Retracted Article: Hydrogen sulfate ion sensing in aqueous media based on a fused pyrimido benzothiazole derivative†
Abstract
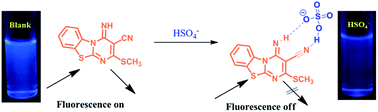
A sensitive and selective receptor 3-cyano-4-imino-2-methylthio-4H-pyrimido[2,1-b][1,3]benzothiazole (SVK-1) bearing a fused pyrimido benzothiazole structure was developed for the recognition of HSO4− anions. UV-vis and fluorescence emission spectroscopy were employed for the recognition for HSO4− over other anions such as Cl−, Br−, I−, F−, AcO−, H2PO4−, ClO4− and NO3− in an aqueous medium.

 Please wait while we load your content...
Please wait while we load your content...