Direct synthesis of N-sulfenylimines through oxidative coupling of amines with disulfides/thiols over copper based metal–organic frameworks†
Wei Longa,
Wenge Qiu*a,
Chuanqiang Lib,
Liyun Songa,
Guangmei Baia,
Guizhen Zhanga and
Hong He*a
aBeijing Key Laboratory for Green Catalysis and Separation, Department of Chemistry and Chemical Engineering, College of Environmental and Energy Engineering, Beijing University of Technology, Beijing 100124, China. E-mail: qiuwenge@bjut.edu.cn
bDepartment of Applied Chemistry, College of Science, Chongqing Jiaotong University, Chongqing 400074, China
First published on 20th April 2016
Abstract
A highly porous metal–organic framework based on supramolecular building blocks with pcu-topology (pcu-MOF) has been tested for the oxidative coupling of amines and disulfides or thiols to afford the N-sulfenylimines directly in good yields without the formation of N-sulfinyl- and N-sulfonylimines. The pcu-MOF catalyst could be easily recovered from the reaction mixture by simple filtration, and could be reused at least five times without any substantial loss in the yield. The control experiments and mechanistic studies suggested that the oxidative coupling process involved the imine formation and the N–S coupling reaction.
Introduction
N-Sulfenylimines, with a functional group of N-sulfur binding imines, H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSR, have been widely used as reagents or intermediates in organic synthesis. For instance, these compounds had been utilized to prepare cyclic nitrile compounds,1 β-lactams,2 tetrahydro-β-carbolines3 and many other biologically organic molecules.4 Therefore, any efficient method for the preparation of sulfenimines would be extremely useful. Although advances in cross-coupling reactions using transition metal-catalysis have led to the development of effective strategies for the formation of nitrogen–sulfur (N–S) bonds,5 efficient and catalytic methods to prepare N-sulfenylimines have remained relatively undeveloped. Usually, N-sulfenimines can be prepared from the condensation of sulfenamides with aldehydes or ketones,6 or from the conversion of ketoximes and secondary nitro compounds.7 In addition, Davis8 reported that sulfenylimines could be synthesized directly through the condensation of disulfides, aldehydes and ammonia in the presence of silver complexes, but a stoichiometric amount of silver complexes was needed. The direct oxidative coupling of amines and thiols often provided sulfonamides due to the susceptivity of substrates under oxidation conditions.9 Very recently, Jang10 group presented an pioneer work on the synthesis of N-sulfenylimines through direct oxidative coupling of amines with thiols in the presence of homogeneous Cu(I) catalyst.
NSR, have been widely used as reagents or intermediates in organic synthesis. For instance, these compounds had been utilized to prepare cyclic nitrile compounds,1 β-lactams,2 tetrahydro-β-carbolines3 and many other biologically organic molecules.4 Therefore, any efficient method for the preparation of sulfenimines would be extremely useful. Although advances in cross-coupling reactions using transition metal-catalysis have led to the development of effective strategies for the formation of nitrogen–sulfur (N–S) bonds,5 efficient and catalytic methods to prepare N-sulfenylimines have remained relatively undeveloped. Usually, N-sulfenimines can be prepared from the condensation of sulfenamides with aldehydes or ketones,6 or from the conversion of ketoximes and secondary nitro compounds.7 In addition, Davis8 reported that sulfenylimines could be synthesized directly through the condensation of disulfides, aldehydes and ammonia in the presence of silver complexes, but a stoichiometric amount of silver complexes was needed. The direct oxidative coupling of amines and thiols often provided sulfonamides due to the susceptivity of substrates under oxidation conditions.9 Very recently, Jang10 group presented an pioneer work on the synthesis of N-sulfenylimines through direct oxidative coupling of amines with thiols in the presence of homogeneous Cu(I) catalyst.
In the past decade, metal–organic frameworks (MOFs) have received much attention as catalytic materials in addition to their applications in gas storage and separation due to their unique features, including their crystallinity, porous structure, and huge specific surface area.11 Many kinds of porous MOFs have been used as catalysts for different reaction systems. For instance, the well-known MOFs, HKUST-1 and MOF-253, have emerged as promising heterogeneous catalysts for C–O,12 C–N13 and C–S14 coupling reactions. In our previous work, a new metal–organic framework based on supramolecular building blocks (SBBs) with pcu-topology,15 denoted as pcu-MOF, were reported, which has high density metal open sites and large open windows. These points encouraged us to explore its application as a heterogeneous catalyst. Herein we reported the direct oxidative coupling of amines and disulfides or thiols to provide various N-sulfenylimines without the formation of N-sulfinyl- and N-sulfonylimines in the presence of pcu-MOF, which can be reused at least five times without a significant degradation in catalytic activity. To the best of our knowledge, this is the first report about a heterogeneous catalytic system for generation of sulfenylimines.
Results and discussion
Synthesis and characterization of the pcu-MOF
The pcu-MOF was prepared by a solvothermal method according to the reported procedure.15 The XRD patterns of the pcu-MOF sample (Fig. 1) matched well with the published results,15 indicating the pure phase of the MOF and the construction of the intended crystalline framework. Nitrogen sorption measurements were carried out to determine the specific surface area and the pore structure of the pcu-MOF sample (Fig. S1, Table S1†). Its BET surface area and adsorption average pore width were 2010 m2 g−1 and 1.94 nm, respectively, demonstrating the porosity and stability of the pcu-MOF after removing the included and coordinated solvents. The SEM image of the pcu-MOF sample showed that the MOF particles presented cubic or polyhedral shapes with sizes in the range of 20–150 μm (Fig. S2†). Thermogravimetric data showed that the pcu-MOF was stable up to 300 °C (Fig. S3†). There was a continuous weight loss of 5.91% from room temperature to 200 °C for the pcu-MOF sample, which was corresponding to the loss of physisorbed H2O and residual ethanol in the MOF channels. The weight loss of 7.75% between 200 and 260 °C could be attributed to the dehydration of coordinated H2O in the pcu-MOF, being close to the calculated weight change (6.73%). | ||
| Fig. 1 X-ray diffraction patterns for pcu-MOF samples: as-synthesized (red), after activation (blue) and used after five catalysis cycles (cyan). | ||
pcu-MOF as a solid catalyst for the oxidative coupling of 4-methoxybenzylamine with diphenyldisulfide
In this work, 4-methoxybenzylamine (1) and diphenyldisulfide (2), were chosen as model substrates. For optimizing the reaction conditions, various solvents and additives were screened under different temperatures in the presence of the pcu-MOF. The experimental results at various conditions were presented in Table 1. It was found that the yields of N-sulfenylimine (3) increased gradually (Table 1, compare entries 2–4) with a rising of temperature in the beginning, indicating the effect of temperature on the coupling reaction, but then the yields of 3 decreased in the range of 120–140 °C. One possible reason was that the substrates was oxidized under high temperature.16 Considering the stability of the reagents and pcu-MOF, all the following experiments were conducted at 120 °C. The desired product was not formed in the absence of pcu-MOF (Table 1, entry 1), implying that the pcu-MOF was necessary for the oxidative coupling reaction of 4-methoxybenzylamine with diphenyldisulfide.| Entry | Catalysts | Solvents | Additives | Temp (°C) | Yieldse (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-methoxylbenzylamine (5 mmol), diphenyldisulfide (1.25 mmol), MOFs (0.125 mmol, based on copper), DTBN (0.25 mmol), TBD (0.25 mmol), solvent (5 mL), for 18 h under O2 atmosphere.b H4L represents 1,1-bis-[3,5-bis(carboxy)phenoxy]methane.c 0.5 mmol additive was added.d The reaction was carried out under nitrogen atmosphere.e Isolated yields.f Data of reaction for 48 h. The symbol of n.r represents no reaction. TBD, DTBN, TEMPO and PINO represent 1,5,7-triazabicyclo[4.4.0]dec-5-ene, di-tert-butylnitroxide, 2,2,6,6-tetramethyl-1-piperidinyloxyl, and N-hydroxyphthalimide, respectively. | |||||
| 1 | — | DMSO | DTBN, TBD | 120 | n.r |
| 2 | pcu-MOF | DMSO | DTBN, TBD | 100 | 52 |
| 3 | pcu-MOF | DMSO | DTBN, TBD | 110 | 57 |
| 4 | pcu-MOF | DMSO | DTBN, TBD | 120 | 68 (93f) |
| 5 | pcu-MOF | DMSO | DTBN, TBD | 130 | 63 |
| 6 | pcu-MOF | DMSO | DTBN, TBD | 140 | 57 |
| 7 | pcu-MOF | DMSO | — | 120 | 12 |
| 8 | pcu-MOF | DMSO | DTBNc | 120 | 35 |
| 9 | pcu-MOF | DMSO | TBDc | 120 | 38 |
| 10 | pcu-MOF | DMSO | TEMPOc | 120 | 23 |
| 11 | pcu-MOF | DMSO | PINOc | 120 | 21 |
| 12 | pcu-MOF | DMSO | Triethylaminec | 120 | 5 |
| 13 | pcu-MOF | Toluene | DTBN, TBD | 120 | 51 |
| 14 | pcu-MOF | PhCl | DTBN, TBD | 120 | 43 |
| 15 | pcu-MOF | Anisole | DTBN, TBD | 120 | 41 |
| 16 | pcu-MOF | NMP | DTBN, TBD | 120 | 49 |
| 17 | pcu-MOF | DMSO | DBNO, TBD, H2O2 | 120 | 65 |
| 18 | pcu-MOF | DMSO | DBNO, TBD, N2d | 120 | n.r |
| 19 | Cu(NO3)2 | DMSO | DTBN, TBD | 120 | 21 |
| 20 | CuI | DMSO | DTBN, TBD | 120 | 83 |
| 21 | Cu(NO3)2 + H4Lb | DMSO | DTBN, TBD | 120 | 2 |
| 22 | HKUST-1 | DMSO | DTBN, TBD | 120 | 23 |
It was found that ligand additives had large effects on the copper-catalyzed oxidation reaction of amines,9a,9c,17 so the effects of several organic additives were examined. In the model reaction, compound 3 was obtained in only 12% yield (Table 1, entry 7) when no additive was added. The use of DTBN, TBD, TEMPO and PINO separately with the loading of 20 mol% resulted in modest increase in the yields of 3 (Table 1, entry 8–11), suggesting the existence of improvements of these additives in the reaction. When DTBN and TBD were used at the same time, 3 was obtained in good yield (Table 1, entry 4), indicating that there might exist a synergistic effect between DTBN and TBD. The control experiment using the same amount of DTBN and TBD in the absence of pcu-MOF gave no product (Table 1, entry 1), suggesting that the additives alone did not have appreciable catalytic activity but served as co-catalyst to promote the activity of pcu-MOF. The addition of triethylamine could not promote the reaction satisfactorily. One possibility is that the interaction of substrate molecules with catalyst was impeded due to the coordination of triethylamine with the copper ion on the MOF.
The effects of solvents were also tested (Table 1, entry 13–16). Reaction in toluene, chlorobenzene, anisole or N-methyl-2-pyrrolidone (NMP), respectively, gave the coupled product in moderate yields. DMSO was found to be the best solvent for the oxidative coupling reaction (Table 1, entry 4). In addition, a good yield of 3 could be obtained when oxygen was replaced by H2O2 (Table 1, entry 17), but no reaction was observed without the oxidant (Table 1, entry 18), implying the importance of the oxidant in the coupling reaction. According to the above results, we conducted the oxidative coupling reaction in DMSO at 120 °C in the presence of DTBN and TBD with the total loading of 20 mol% as the standard reaction condition. The coupling reaction could afford 3 in 93% yield for 48 h with complete conversion of diphenyldisulfide (Fig. 2) without any N-sulfinyl- and N-sulfonylimines.
In order to determine whether the catalysis of pcu-MOF is truly heterogeneous or, on the contrary, is due to some leached copper species present in the reaction solution, a control experiment was performed: the pcu-MOF catalyst was removed from hot solution by filtration after 4 h. The filtrate was further performed for another 44 h. No significant catalytic conversion was observed (Fig. 2), indicating that the reaction was terminated after removal of the MOF catalyst. Furthermore, inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis of the filtrate showed that there was only about 1.5 ppm of copper ions in the solution, which corresponds to 0.006% of copper ions in the solution after one cycle of the oxidative coupling reaction. These results clearly confirmed that the catalysis of pcu-MOF is heterogeneous in nature, and the contribution of leached copper species soluble in the solution was negligible. As a result, the open copper sites on the axis of paddle-wheel SBUs within the pcu-MOF might be responsible for the promotion of the oxidative coupling reaction.18
On the other hand, the catalytic activity of Cu(NO3)2 and CuI in homogeneous solution was studied in the presence of DTBN and TBD for the same reaction (Table 1, entries 19 and 20). The experimental results showed that both Cu(I) and Cu(II) could improve the oxidative coupling reaction, but the yield of 3 in the presence of CuI was much higher than that of Cu(NO3)2, revealing the high activity of Cu(I).19 A widely used MOF, namely CuBTC20 (Fig. S4 and S5†) that was constructed from paddle-wheel type copper clusters and 1,3,5-benzenetricarboxylate molecules, was also tested, but it showed a low activity for the oxidative coupling reaction between 4-methoxylbenzylamine and diphenyldisulfide (Table 1, entry 22). The yield of the desired product in homogeneous catalytic process by both Cu(NO3)2 and H4L (Table 1, entry 21) was much lower than that of the heterogeneous system of pcu-MOF. Owing to the same amount of copper species being used in the heterogeneous and homogeneous reaction systems, this result further revealed that the high density of open copper sites within the pcu-MOF were the active sites of pcu-MOF catalyst.
The pcu-MOF catalyst demonstrated good reusability for the oxidative coupling reaction of 4-methoxylbenzylamine and diphenyldisulfide (Fig. 3). The yields of compound 3 were 68%, 69%, 67%, 65% and 67%, respectively, in successive cycles for 18 hours. The image (Fig. S2b†) and the nitrogen physisorption measurement (Fig. S1†) demonstrated the porosity and stability of pcu-MOF after five reaction runs. The BET surface area and pore volume of the reused catalyst decreased slightly from 2010 to 1743 m2 g−1 and 0.979 to 0.929 cm3 g−1 (Table S1†), respectively, probably due to the adsorption of a few reactant or product molecules in the MOF cavities.21,22 The main diffraction peaks of the pcu-MOF catalyst after five reaction runs also matched well to the data of fresh catalyst (Fig. 1). The decrease in peaks intensities and few diffraction peaks becoming broaden can be attributed to the degradation of partial crystal particles induced by the stirring and the effect of reaction medium (Fig. S2b†).
Generality of the oxidative coupling reaction
With the optimal reaction conditions in hand, we then investigated the substrate scope of amines and thiols. As shown in Table 2, the reaction proceeded smoothly with substrates containing electron-drawing groups or electron-donating groups in moderate yields (Table 2, entry 2–6), demonstrating that this reaction has a high degree of functional group tolerance. Comparing to 4-methoxylbenzylamine, 3-methoxylbenzylamine could also react smoothly with diphenyldisulfide and gave a high yield of the desired product (Table 2, entry 1). When thiophenols were used as the sulfur resource, the coupling reaction performed well too (Table 2, entry 7–9). In addition, one could find that steric effect had influence on the transformation as confirmed by the relative lower yield for the α-methyl and α-ethyl substituted substrates (Table 2, entry 11 and 12) than the unsubstituted substrate (Table 1, entry 4). However, the α-phenyl substituted substrate (Table 2, entry 10) was suitable for the smooth conversion. This could be attributed to the high stability of the imine intermediate that would be discussed in the mechanism discussion. When benzenamine, aliphatic amine or alicyclic amine was used, no product was detected (Table 2, entry 14 and 15), or the yield was very low for 48 h (Table 2, entry 16), revealing that the benzylamine group was essential for the oxidative coupling reaction.| Entry | Amines | Sulfur sources | Yieldb (%) |
|---|---|---|---|
| a Reaction condition: benzylamines (5 mmol), diphenyldisulfide/thiophenol (1.25/2.5 mmol), MOFs (0.125 mmol, based on copper), DTBN (0.25 mmol), TBD (0.25 mmol), solvent (5 mL), for 18 h under O2 atmosphere.b Isolated yields based on the sulfur resource.c Data for 48 h. The symbol of n.r represents no reaction. | |||
| 1 |  |
PhSSPh | 65 |
| 2 | 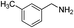 |
PhSSPh | 42 |
| 3 |  |
PhSSPh | 53 |
| 4 | 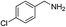 |
PhSSPh | 34 |
| 5 |  |
PhSSPh | 39 |
| 6 |  |
PhSSPh | 38 |
| 7 | 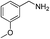 |
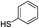 |
69 |
| 8 |  |
 |
53 |
| 9 |  |
 |
51 |
| 10 | 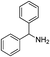 |
PhSSPh | 58 |
| 11 |  |
PhSSPh | 39 |
| 12 |  |
PhSSPh | 43 |
| 13 |  |
PhSSPh | 65 |
| 14 | 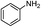 |
PhSSPh | n.r |
| 15 |  |
PhSSPh | n.r |
| 16 |  |
PhSSPh | 11c |
Mechanistic considerations
Copper-catalyzed reaction usually occurred via a single electron transfer process or an organometallic pathway.23 Several strategies involving copper mediated N–S bond formation have been reported.5 For these homogeneous Cu(I) or Cu(II) catalyzed processes, the proposed mechanisms included the coordination of thiol and amine to copper atom as well as the oxidative formation of a Cu–S bond and a Cu–N bond.5,9c In order to understand the mechanism of the present catalytic system, several control experiments were performed. Only a trace amount of product (compound 3) was observed when the oxidative coupling reaction of 4-methoxylbenzylamine and diphenyldisulfide was conducted under nitrogen atmosphere, suggesting that oxygen is necessary for the present procedure. However, a radical pathway could be ruled out because stoichiometric radical scavenger (TEMPO) did not inhibit the reaction at all.24 In the absence of diphenyldisulfide, a high yield of 4-methoxybenzaldehyde was detected (Table S2†), suggesting that imine derivatives might act as the intermediate formed by the oxidation of the corresponding amine under air conditions.25 The formation of 4-methoxybenzaldehyde was due to hydrolysis of the imine during work-up.26 The imine intermediate mechanism was further supported by the experiment, in which the coupling reaction could afford the desired product, N-(phenylthio)-diphenylmethanimine, in a good yield of 65% when diphenylmethylimine was used as the substrate (Table 2, entry 13). These results suggested that the oxidative coupling reaction might carry out in a two steps mechanism involving the imine formation by amine oxidation and the N–S coupling reaction.To gain further insights into the mechanism, the function of the additives in each step was also studied. In the oxidation reaction of 4-methoxylbenzylamine (Table S2†), the yield of oxidation product in the presence of DTBN was higher than that in the absence of additive, indicating the promotion of DTBN to the imine formation due to its activation of molecular oxygen.27 But TBD showed no effect on the oxidation of benzylamine. For the coupling reaction of diphenylmethylimine and diphenyldisulfide, only 5% product was afforded when no additive was added, however, the desired coupling product produced a yield of 57% or 59% in the presence of DTBN or TBD, showing that the additives had significant effect on the coupling reaction. Here both DTBN and TBD might act as bases. Only 8% yield of N-(phenylthio)-diphenylmethanimine was obtained when the reaction was carried out under nitrogen atmosphere, implying that oxygen also played an important role in the N–S coupling step.
To investigate the function of pcu-MOF in the oxidation coupling reaction, the electron paramagnetic resonance (EPR) spectra of pure pcu-MOF, pcu-MOF with 4-methoxybenzylamine, pcu-MOF with diphenyldisulfide, and pcu-MOF in the reaction system at X-band (9.06 GHz) were recorded at 90 °C, respectively. The spectra of the reaction system at X-band were dominated by a broad central line at g = 2.112, (Fig. 4), which could be attributed to Cu2+–Cu2+ dimmers or mononuclear Cu2+ ions.28 Compared to the spectra of pure pcu-MOF in toluene (Fig. S6a†), the decrease of the intensity of EPR signal of the reaction mixture with time and the change of g-value indicated the occurrence of interaction between reaction substrates and copper sites. However, different phenomena were observed when 4-methoxybenzylamine and diphenyldisulfide existed with pcu-MOF separately. For the system of pcu-MOF and 4-methoxybenzylamine, the g-value decreased from 2.147 to 2.115, indicating that 4-methoxybenzylamine coordinated to the Cu(II) ions on the framework that resulted in a shift of the g-parameters to lower values,29 but the intensity of EPR signal remained (Fig. S6b†). On the contrary, for the system of pcu-MOF and diphenyldisulfide, the intensity of EPR signal decreased obviously with time, but no change of the g-value was observed (Fig. S6c†). These results revealed the different interaction between the two reagents and the copper ions on the MOF framework. The intensity of the EPR signal of the reaction system decreased with time, indicating that part of the Cu(II) ions were reduced by the disulfide. It has been reported that reductive reagent or in situ generated reductive species could reduce the Cu(II) on the MOF framework to Cu(I) species, which can be oxidized into Cu(II) by O2 oxidation at the end of the reaction without affecting heterogeneous nature of the MOF catalyst.30
Based on the literature10,30 and our experimental results, a plausible mechanism of the oxidative coupling reactions was outlined in Scheme 1. At first, benzylamine interacted with Cu(II) ion on the pcu-MOF framework to form benzylamine–Cu complex, which underwent oxidation to afford imine intermediate. Meanwhile, disulfide interacted with Cu(II) ion, leading to the reduction of Cu(II) to Cu(I), which in turn initiated the activation of disulfide. Then imine and the activated disulfide coupled together to afford the product N-sulfenimine.
Conclusions
In summary, we have shown for the first time that copper-based MOF is an efficient heterogeneous catalyst for the oxidative coupling of amines and disulfides or thiols. This protocol provides a novel and direct synthesis of N-sulfenylimines in good yields without the formation of N-sulfinyl- and N-sulfonylimines. The experimental results prove that pcu-MOF can be reused at least five times without any degradation in catalytic activity. Based on the control experiments and the characterization data, a plausible two steps mechanism was proposed, which involved the imine formation by amine oxidation and the N–S coupling reaction. The open copper sites on the axis of paddle-wheel SBUs within pcu-MOF are responsible for the both two steps.Experimental
Materials and instruments
All solvents and chemicals were obtained commercially and used as received without further purification. The pcu-MOF15 and CuBTC20 were synthesized according to the reported procedures, respectively. Powder X-ray diffraction was performed on a Bruker D8 Advance X-ray diffractometer using Cu-Kα radiation (λ = 1.5406 Å) at room temperature with a scan speed of 0.5 s per step and a step size of 0.02°. The images of the MOF samples were conducted on Hitachi S 4800 scanning electron microscope (SEM) at room temperature. 1H-NMR and 13C-NMR data were collected on Bruker ARX-400 or Bruker ARX-600 spectrometers with DMSO-d6 solution by using tetramethylsilane as an internal standard. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis was performed on an IRIS Intrepid ER/S (Thermo Elemental) instrument. Nitrogen sorption measurement was performed at −196 °C on a Micromeritics ASAP 2020. A sample of approximately 50 mg was outgassed at 150 °C for 12 h and then nitrogen isotherm at −196 °C was measured in liquid nitrogen bath using UHP-grade (99.999%) gas source. The Brunauer–Emmett–Teller (BET) surface areas of pcu-MOF and CuBTC were calculated based on the nitrogen absorption isotherms. The continuous wave (CW) EPR spectra of pure pcu-MOF, pcu-MOF with 4-methoxybenzylamine, pcu-MOF with diphenyldisulfide, and pcu-MOF in the reaction system in toluene at X-band were measured using a JES-FA200 spectrometer (microwave frequency 9.06 GHz, power of the microwave 0.998 mW) at 90 °C. Thermogravimetric analysis (TGA) was performed on an NETZSCH TG 209F3 instrument under nitrogen atmosphere (250 mL min−1). The sample was heated at a constant rate of 10 °C min−1 from 40 °C to 600 °C.General procedure for the pcu-MOF catalyzing the oxidative coupling reaction
The oxidative coupling reaction was performed as follows. In a typical procedure, pcu-MOF (33.5 mg, 0.125 mmol), di-tert-butylnitroxide (DTBN, 36.0 mg, 0.25 mmol) and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD, 35.0 mg, 0.25 mmol) were added to a solution of 4-methoxybenzylamine (685 mg, 5.0 mmol) and diphenyldisulfide (261 mg, 1.25 mmol) in DMSO (5 mL). A slow stream of O2 was passed through the solution for 10 min. Then, the reaction mixture was stirred at 120 °C for 18 h under O2 atmosphere. The solvent was removed under vacuum, and the residue was purified by flash silica gel column chromatography by using 1% ethyl acetate/hexane as an eluent. The fractions were collected and evaporated to afford the pure desired product.Recycling experiments
pcu-MOF (310 mg, 1.25 mmol), di-tert-butyl nitroxide (DTBN, 360 mg, 2.5 mmol) and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD, 350 mg, 2.5 mmol), 4-OMe-benzyl amine (6850 mg, 50 mmol), phenyl disulfide (2610 mg, 12.5 mmol) and DMSO (50 mL) were added into a three-neck flask (250 mL) with a magnetic bar, and then the tube was vacuumed and purged with oxygen three times before it was finally pressurized with double oxygen balloons. Subsequently, the flask was stirred at 120 °C for 18 h. After completion of the reaction, the solid catalyst was recovered by filtration, washing with hot methanol and drying under vacuum to remove the residual solvent, and reused for the next run. The liquid mixture was collected, and the solvent was removed under vacuum. The residue was purified by flash silica gel column chromatography by using 1% ethyl acetate/hexane as an eluent, fractions were collected and evaporated to afford the N-(4-methoxybenzylidene)-S-phenylthiohydroxylamine.Acknowledgements
We acknowledge the financial support of the national natural science foundation of China (Grant No. 21577005, 21307168 and 21277009).References
- J. Boivin, E. Fouquet and S. Z. Zard, J. Am. Chem. Soc., 1991, 113, 1055–1057 CrossRef CAS.
- (a) S. Coantic, D. Mouysset, S. Mignani, M. Tabart and L. Stella, Tetrahedron, 2007, 63, 3205–3216 CrossRef CAS; (b) D. A. Burnett, D. J. Hart and J. Liu, J. Org. Chem., 1986, 51, 1929–1930 CrossRef CAS.
- M. J. Wanner, R. N. S. van der Haas, K. R. de Cuba, J. H. van Maarseveen and H. Hiemstra, Angew. Chem., Int. Ed., 2007, 46, 7485–7487 CrossRef CAS PubMed.
- (a) L. Craine and M. Raban, Chem. Rev., 1989, 89, 689–713 CrossRef CAS; (b) J. A. Ellman, T. D. Owens and T. P. Tang, Acc. Chem. Res., 2002, 35, 984–995 CrossRef CAS PubMed.
- (a) H. Kim, S. H. Kwak, G. Lee and Y. Gong, Tetrahedron, 2014, 70, 8737–8743 CrossRef CAS; (b) Z. Wang, Y. Kuninobu and M. Kanai, J. Org. Chem., 2013, 78, 7337–7342 CrossRef CAS PubMed; (c) N. Taniguchi, Synlett, 2007, 12, 1917–1920 CrossRef; (d) R. Paul and T. Punniyamurthy, RSC Adv., 2012, 2, 7057–7060 RSC; (e) A. Correa, I. Tellitu, E. Dominguez and R. Sanmartin, Org. Lett., 2006, 8, 4811–4813 CrossRef CAS PubMed.
- (a) J. J. Damico, J. Org. Chem., 1961, 26, 3436–3445 CrossRef CAS; (b) J. C. Foster and J. B. Matson, Macromolecules, 2014, 47, 5089–5095 CrossRef CAS; (c) J. C. Foster, C. R. Powell, S. C. Radzinski and J. B. Matson, Org. Lett., 2014, 16, 1558–1561 CrossRef CAS PubMed.
- (a) K. A. Lukin and B. A. Narayanan, Tetrahedron, 2002, 58, 215–219 CrossRef CAS; (b) J. Bures, C. Isart and J. Vilarrasa, Org. Lett., 2007, 9, 4635–4638 CrossRef CAS PubMed; (c) J. Esteban, A. M. Costa, F. Urpi and J. Vilarrasa, Tetrahedron Lett., 2004, 45, 5563–5567 CrossRef CAS.
- (a) F. A. Davis, W. A. R. Slegeir and S. Evans, J. Org. Chem., 1973, 38, 2809–2813 CrossRef CAS; (b) F. A. Davis, W. A. R. Slegeir and J. M. Kaminsky, J. Chem. Soc., Chem. Commun., 1972, 634–635 RSC.
- (a) X. Huang, J. Wang, Z. Ni, S. Wang and Y. Pan, Chem. Commun., 2014, 50, 4582–4584 RSC; (b) K. Bahrami, M. M. Khodaei and M. Soheilizad, Tetrahedron Lett., 2010, 51, 4843–4846 CrossRef CAS; (c) N. Taniguchi, Eur. J. Org. Chem., 2010, 2670–2673 CrossRef CAS.
- C. Lee, X. Wang and H. Jang, Org. Lett., 2015, 17, 1130–1133 CrossRef CAS PubMed.
- (a) A. Corma, H. Garcia and F. X. Llabresi Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed; (b) A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Catal. Sci. Technol., 2011, 1, 856–867 RSC; (c) A. Dhakshinamoorthy, M. Opanasenko, J. Cejka and H. Garcia, Catal. Sci. Technol., 2013, 3, 2509–2540 RSC.
- M. Wang, B. Z. Yuan, T. M. Ma, H. F. Jiang and Y. W. Li, RSC Adv., 2012, 2, 5528–5530 RSC.
- N. T. S. Phan, T. T. Nguyen, C. V. Nguyen and T. T. Nguyen, Appl. Catal., A, 2013, 457, 69–77 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro, P. Concepcion and H. Garcia, Catal. Commun., 2011, 12, 1018–1021 CrossRef CAS.
- C. Li, W. Qiu, W. Shi, H. Song, G. Bai, H. He, J. Li and M. J. Zaworotko, CrystEngComm, 2012, 14, 1929–1932 RSC.
- (a) R. D. Patil and S. Adimurthy, Adv. Synth. Catal., 2011, 353, 1695–1700 CrossRef CAS; (b) T. Sonobe, K. Oisaki and M. Kanai, Chem. Sci., 2012, 3, 3249–3255 RSC.
- (a) N. Taniguchi, Synlett, 2007, 12, 1917–1920 CrossRef; (b) F. Chen, G. Liao, X. Li, J. Wu and B. Shi, Org. Lett., 2014, 16, 5644–5647 CrossRef CAS PubMed; (c) F. G. Xi, Y. Yang, H. Liu, H. F. Yao and E. Q. Gao, RSC Adv., 2015, 5, 79216–79223 RSC.
- L. Alaerts, E. Seguin, H. Poelman, F. Thibault-Starzyk, P. A. Jacobs and D. E. De Vos, Chem.–Eur. J., 2006, 12, 7353–7363 CrossRef CAS PubMed.
- M. Largeron and M. B. Fleury, Angew. Chem., Int. Ed., 2012, 51, 5409–5412 CrossRef CAS PubMed.
- K. Schlichte, T. Kratzke and S. Kaskel, Microporous Mesoporous Mater., 2004, 73, 81–88 CrossRef CAS.
- L. H. Wee, S. R. Bajpe, N. Janssens, I. Hermans, K. Houthoofd, C. E. A. Kirschhock and J. A. Martens, Chem. Commun., 2010, 46, 8186–8188 RSC.
- C. Q. Li, W. G. Qiu, W. Long, F. Deng, G. M. Bai, G. Z. Zhang, X. H. Zi and H. He, J. Mol. Catal. A: Chem., 2014, 393, 166–170 CrossRef CAS.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed.
- Y. Sun, W. Wu and H. Jiang, Eur. J. Org. Chem., 2014, 4239–4243 CrossRef CAS.
- (a) B. Huang, H. Tian, S. Lin, M. Xie, X. Yu and Q. Xu, Tetrahedron Lett., 2013, 54, 2861–2864 CrossRef CAS; (b) D. Damodara, R. Arundhathi and P. R. Likhar, Adv. Synth. Catal., 2014, 356, 189–198 CrossRef CAS.
- P. Capdevielle, A. Lavigne and M. Maumy, Tetrahedron, 1990, 46, 2835–2844 CrossRef CAS.
- D. L. Kleyer, R. Curtis Haltiwanger and T. H. Koch, J. Org. Chem., 1983, 48, 147–152 CrossRef CAS.
- (a) H. E. L. Mkami, M. I. H. Mohideen, C. Pal, A. McKinlay, O. Scheimann and R. E. Morris, Chem. Phys. Lett., 2012, 544, 17–21 CrossRef; (b) M. Simenas, M. Kobalz, M. Mendt, P. Eckold, H. Krautscheid, J. Banys and A. Poppl, J. Phys. Chem. C, 2015, 119, 4898–4907 CrossRef CAS.
- P. Valvekens, E. D. Bloch, J. R. Long, R. Ameloot and D. E. D. Vos, Catal. Today, 2015, 246, 55–59 CrossRef CAS.
- (a) V. Mahendran and S. Shanmugam, RSC Adv., 2015, 5, 20003–20010 RSC; (b) S. Parshamoni, S. Sanda, H. S. Jena and S. Konar, Dalton Trans., 2014, 43, 7191–7199 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01971d |
| This journal is © The Royal Society of Chemistry 2016 |





