Promotional effect and mechanism study of nonmetal-doped Cr/CexTi1−xO2 for NO oxidation: tuning O2 activation and NO adsorption simultaneously†
Lei Zhongac,
Qin Zhong*abc,
Wei Caibc,
Shen Zhangb,
Yang Yubc,
Man Oubc and
Fujiao Songbc
aSchool of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, PR China. E-mail: zq304@mail.njust.edu.cn; Fax: +86 25 84315517; Tel: +86 25 84315517
bSchool of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, PR China
cNanjing AIREP Environmental Protection Technology Co., Ltd, Jiangsu, Jiangsu 210091, PR China
First published on 2nd February 2016
Abstract
Nonmetal-doped Cr/CexTi1−xO2 catalysts were evaluated for selective catalytic oxidation (SCO) of NO, and these were synthesized by using cyanamide as a nonmetal source. The aim of this paper was to elucidate the detailed composition–structure–property relationships. The characterization results demonstrated that the optimized performance was correlated with the formation of superoxide radicals, which was derived from the nonmetal doping and confirmed by EPR studies. H2-TPR and O2-TPD experiments indicated that the addition of cyanamide was beneficial to tune O2 activation and improve NO adsorption strength simultaneously. XPS results suggested that N species were successfully incorporated into the lattice of a cerium–titanium solid solution and substituted for oxygen. Additionally, the designed FTIR and Raman measurements were applied to identify the doping sites, that is, N species were inclined to substitute the O atoms around cerium to form the Ce–N–Ti and Ce–N–Ce bonds. Finally, the catalytic mechanism was tentatively proposed based on the analysis of in situ DRIFTS results.
1. Introduction
Air pollution is becoming more serious with the development of industry. As a result, the emission control of atmospheric pollutants is extremely urgent in order to meet the strict environmental regulations. Nitrogen oxides (NOx), originating from coal-fired flue gas emission, are the new and focused objects of governance in China, and are associated with acid deposition, photochemical smog and ozone depletion.1,2 Development of effective methods to control nitric oxide (NO) emission has been proposed as a prospective research area because NO is the main source of NOx. Current popular techniques for NOx removal are selective catalytic reduction (SCR)3,4 and NOx storage and reduction (NSR),5,6 which both convert NOx into N2. This problem is addressed through the increase of the NO oxidation rate, because NO2 is much easier to be either reduced into nitrogen or absorbed by alkaline solution.7,8 Hence, the technology of selective catalytic oxidation, transforming NO into NO2, plays a crucial role in the current techniques. However, the oxidation rate of NO under the normal condition is too slow to meet the process requirements. In addition, the removal efficiency will be the optimum if the ratio of NO/NOx equals to 60%.9 Therefore, considerable attention has been focused on developing the eligible catalysts for NO oxidation in recent years.10–12The appropriate transition metal oxide catalysts can replace the noble metal catalysts due to their potential practicality, low cost and high catalytic efficiency. Among them, Cr-based catalysts could act as the complete oxidation metal oxide, which is due to its Cr3+/Cr6+ redox couple and strong ability of absorbing and activating acid gas.13–15 Moreover, the deposition precipitation method is proven to be a very effective preparation method.12,16 Recently, ceria, involving the Ce4+/Ce3+ couple, could act as an oxygen buffer by releasing-uptaking oxygen through redox processes, which is widely applied in environmental catalytic field.17 The structure modification of ceria lattice by doping with foreign metal cations, such as Ti4+, might increase the specific surface area, enhance the redox behavior and improve the thermal stability effectively. Hence, ceria–titanium solid solution (CexTi1−xO2) is considered as the ideal oxide, which has been extensively studied for NOx emission control.18,19 The Ce–O–Ti short-range order species with the interaction between Ce and Ti in atomic scale has been proven to be the active site.20,21 In order to improve the catalytic performance, great attention has been paid to modify the material, such as nonmetal doping.22–26 The positive effect of nonmetal doping depends on many factors such as the dopant concentration, the distribution of the dopant, the configuration of doping ions and so on.22 However, the study of nonmetal doped on the Ce–O–Ti bond is rarely reported. Currently, the study of two non-metallic elements doped TiO2 are still controversial on the doping element. Sullivan et al.27 focus on the study of carbon-doped TiO2 in the presence of melamine borate. Virkutyte and Zhao et al.28,29 found that TiO2 doping with N-rich melamine produced a stable, active and visible light sensitized nanocatalyst. Therefore, considering the particularity of ceria–titanium solid solution, the research of nonmetal doping using two non-metallic material as the dopant is necessary.
In the present work, a series of Cr/CexTi1−xO2 catalysts with the cyanamide as the dopant were prepared by a combination of sol–gel and deposition–precipitation methods. XRD, FTIR, H2-TPR, O2-TPD, XPS, Raman, EPR and in situ DRIFTS are applied to explore the promotional effect of nonmetal doping on the redox performances and the activation ability of reaction atmosphere, and then further to confirm the doping element and sites. Furthermore, on the basis of the detailed analysis of surface-bound species as a function of reaction temperature and reaction time, a possible catalytic mechanism of NO oxidation is proposed.
2. Experimental
2.1 Preparation of the catalysts
All the chemicals were purchased from Alfa Aesar and used as received without further purification. The water used throughout all experiments was purified through a Millipore system. The ceria–titanium solid solution (CexTi1−xO2, abbreviated as CeTi) with equal molar ratio was prepared by the sol–gel method. The typical preparation procedure was described as follows: 0.0176 mol tetrabutyl tianate (C16H36O4Ti) and 0.0176 mol Ce(NO3)3·6H2O were added to 0.0704 mol of acetylacetone (C5H8O2) under continuous stirring. Then, a certain amount of cyanamide (CH2N2) (0, 0.0211 mol, 0.0422 mol and 0.0633 mol) was diluted with 50 mL ethanolic solution at 50 °C and then was added dropwise into the above solution. In this way, the CeTi–C(x) (x represents the cyanamide/CexTi1−xO2 molar ratio) were prepared, i.e., x = 0.6, 1.2, 1.8, which were denoted as CeTi–C(0.6), CeTi–C(1.2), CeTi–C(1.8), respectively. Undoped CeTi was obtained without addition of cyanamide (CH2N2), which was denoted as CeTi–C(0). After stirring evenly, the solution was heated at 80 °C for 4 h in a water bath and dried at 120 °C for 6 h. Then the dried materials were calcined in flowing air at 550 °C for 5 h at a heating rate of 2 °C min−1.In the next step, the loading of chromium oxide was prepared by deposition–precipitation (DP) method. 0.866 g Cr (NO3)3·9H2O was dissolved in deionized water with stirring until completely dissolved. Secondly, a certain amount of NH3·H2O was slowly dropped into the above solution under vigorous stirring at room temperature until the pH of the solution reached 8. Thirdly, 1 g CeTi–C(x) was added. After continuously stirred for 3 h, the precipitate was aged in air for 4 h in the mother solution. After refluxed in the water-bath at 60 °C for 4 h, the obtained sample was dried in the dry oven at 120 °C and then calcined at 500 °C for 4 h to obtain the samples. Moreover, the total content of metal oxide in all of these samples was fixed at 13 wt% of the catalysts.
2.2 Catalytic activity test
The catalytic performance of NO oxidation was tested in a fixed-bed flow micro reactor at atmospheric pressure. Typically, 300 mg sample (sieve fraction of 60–80 mesh) was placed in a quartz reactor (i.d. 6.8 mm). The simulated reactant mixture (400 ppm NO, 8% O2, N2 balance) was fed to the reactor with a total gas flow rate of 100 mL min−1, corresponding to the gas hourly space velocity (GHSV) of 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 h−1. Before the measurement of catalytic activity, each sample was pretreated for 2 h in order to avoid errors caused by NO adsorption. All the catalysts were kept on stream at each temperature for 1 h. The concentrations of inlet and outlet mixture, including NO, NO2, O2, were monitored by the Ecom-JZKN 12 flue gas analyzer (made in Germany). The exit gas from the micro reactor passed through a trap containing the concentrated alkaline solution and then vented out. The NO conversion to NO2 and NO2 selectivity were defined as follows:
400 h−1. Before the measurement of catalytic activity, each sample was pretreated for 2 h in order to avoid errors caused by NO adsorption. All the catalysts were kept on stream at each temperature for 1 h. The concentrations of inlet and outlet mixture, including NO, NO2, O2, were monitored by the Ecom-JZKN 12 flue gas analyzer (made in Germany). The exit gas from the micro reactor passed through a trap containing the concentrated alkaline solution and then vented out. The NO conversion to NO2 and NO2 selectivity were defined as follows:| NO conversion = (NO(in) − NO(out))/NO(in) × 100% | (1) |
| NO2 selectivity= (NO2(out) − NO2(in))/(NO(in) − NO(out)) × 100% | (2) |
2.3 Characterizations of the catalysts
X-ray powder diffraction (XRD) patterns for the samples were recorded in the 2θ range of 5–80° using nickel-filtered Cu Kα radiation (λ = 0.1542 nm). The working voltage of the X-ray tube was 36 kV, and the current was 20 mA. The scanning speed was 8° min−1 and the step value was 0.04°. (Beijing Purkinje General Instrument Co., Ltd, China).The data of Fourier transform infrared spectroscopy (FTIR) were recorded on a Nicolet IS10 spectrometer from 400 to 4000 cm−1 at room temperature on KBr mulls.
Temperature-programmed reduction of hydrogen (H2-TPR) was measured using an on-line thermal conductivity detector (TCD) on Quanta Chembet (3000). 100 mg catalyst was pre-treated in air stream at 300 °C and then cooled down to 50 °C in the same atmosphere. The H2–N2 mixture (10% H2 by volume) was switched on at a flowing rate of 70 mL min−1 and the temperature was increased linearly at a rate of 10 °C min−1.
Temperature-programmed desorption of oxygen (O2-TPD) was conducted on a Micrometritics 2920 Autochem II V4.01 analyzer. About 100 mg of sample was used. After O2 saturation in 1 h, the gas was switched to He for 0.5 h. Subsequently, the temperature was increased linearly to the desired temperature at a rate of 10 °C min−1 in He. Desorption of O2 were both detected by an on-line thermal conductivity detector (TCD).
X-ray photoelectron spectra (XPS) were performed on a Thermo Scientific ESCALAB 250 (UK) apparatus with Al Kα X-rays (hν = 1486.6 eV) as radiation source operated at 150 W. The samples were compensated for charging with low-energy electron beam, and the peak of C 1s (binding energy = 284.4 eV) was used to correct for sample charging.
Visible-Raman spectra were recorded on a Aramis (Horiba Jobin Yvon S.A.S.) Raman Microscope with Ar+ radiation (532 nm laser) equipped with a CCD detector. The laser light was focused onto the samples by using a microscope equipped with a ×50 objective lens.
The electron paramagnetic resonance (EPR) measurements were made at room temperature using a Bruker EMX-10/12-type spectrometer (∼9.7 GHz) in the X-band.
In situ diffused reflectance infrared Fourier transform spectroscopy (in situ DRIFTS) spectra were carried out by a Nicolet IZ10 FTIR spectrometer, equipped with a liquid-nitrogen-cooled MCT detector. 32 scans were averaged for each spectrum, which were recorded at a spectral resolution of 4 cm−1. The DRIFTS cell was filled with KBr (spectral purity) and the background spectra was recorded. Prior to each experiment, the fine catalyst powder was pretreated at 320 °C in high purified N2 for 2 h to eliminate surface impurity. After cooling to ambient temperature, the sample was exposed to a controlled stream of N2–NO–O2 (1% of NO and 2% of O2 by volume) at a rate of 10 °C min−1 for 60 min to be saturated. The adsorption spectra were collected by subtraction of the corresponding background reference.
3. Results and discussion
3.1 Catalytic activity tests
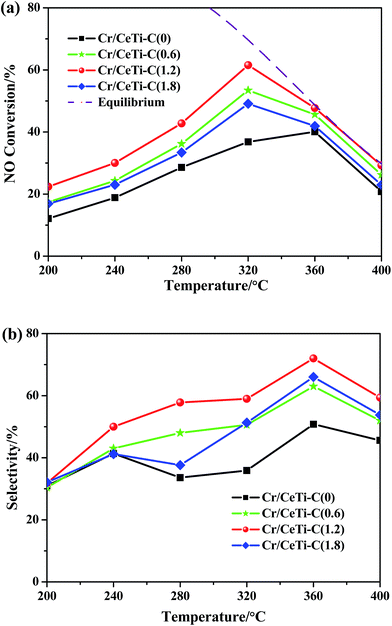
The catalytic activities over the Cr/CeTi–C(x) catalysts with different molar ratios at a temperature range of 200–400 °C were tested, which is displayed in Fig. 1a. For all catalysts, the activity at above 320 °C was thermodynamic controlled, thus the conversion was followed the equilibrium line (in dashed). Besides, in kinetic controlled region, the catalytic performance decreases in the following order: Cr/CeTi–C(1.2) > Cr/CeTi–C(0.6) > Cr/CeTi–C(1.8) > Cr/CeTi–C(0). It can be concluded the catalytic performance of the nonmetal-doped samples are superior to that of the undoped sample. Especially for the Cr/CeTi–C(1.2), the NO conversion reaches 61.5% at 320 °C. However, excessive doping plays a negative role in the activity. These results suggest that an appropriate amount of nonmetal doping is conductive to improve the activity of the Cr/CeTi–C(x) catalyst. Moreover, the catalytic selectivities are also displayed in Fig. 1b, which also confirms the promotional effect of nonmetal doping.3.2 Structural characteristics (XRD and FTIR)
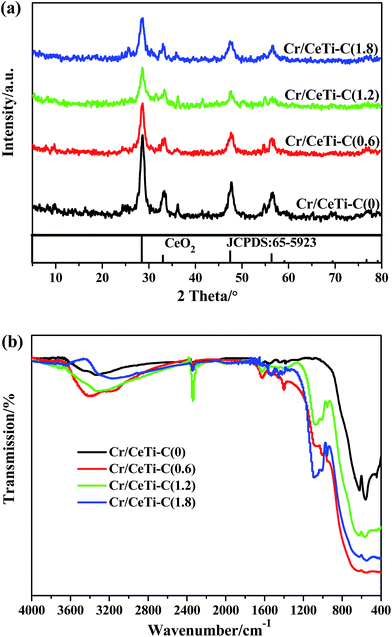
Wide angle XRD patterns were recorded to characterize the phase structure of the products. The results of Cr/CeTi–C(x) catalysts with different molar ratios are shown in Fig. 2a. Only the broad diffraction lines, attributed to cubic fluorite-type phase CeO2 (PDF-ICDD 65-5923), are detected, and no characteristic lines assigned to TiO2 are detected. In many cases, ceria, rather than titania, has been proven to possess more vacancies for the redox reaction.30 Moreover, the crystallinity is significantly suppressed with the increase of the doping amount. It is due to the crystallite nanodimensions effect.31,32 The radius of nonmetal is similar to that of oxygen, hence the incorporation of nonmetal leads to contract and distort of the lattice.24 Thereby, the above difference in lattice strain may be related to surface effects and/or the concentration of oxygen vacancy in these samples.32Since the information of doping component could not be provided by powder XRD, FTIR investigation was employed to provide the valuable information on the textural structure. The FTIR transmission spectra of as-prepared samples were also acquired for comparison, which are shown in Fig. 2b. Several obvious differences are observed. From high to low wave numbers, four band regions located at 3600–2800 cm−1, 1700–1300 cm−1, 1200–800 cm−1 and 720–400 cm−1 are detected. According to the literature, the peaks at 3600–2800 cm−1 are related to the stretching vibration of OH groups from the adsorbed water on the catalysts surface.32,33 The bands at 1700–1300 cm−1 are associated to the N–C stretching modes,34 which are caused by the different doping level. The peaks at 1200–800 cm−1 are considered as the bending vibration of the substituted –OH in CeOH,35,36 and the bands at 720–400 cm−1 are ascribed to the formation of Ce–O–Ti linkage bonds.34 Hence, it comes to the conclusion that nonmetal have successfully been doped in the fine structure of CeTi.
Taken together, the XRD and FTIR results demonstrate the nonmetal element is successfully doped. Importantly, it is discovered that the replacement of oxygen by the non-metallic elements with similar radius is effective. In order to further investigate the effect of nonmetal doping, some other characterizations are used to analyze the physical and chemical properties of the catalysts.
3.3 Redox property (H2-TPR)
Aiming to further evaluate the redox properties of these catalysts, the H2-TPR result is presented in Fig. 3. There are two peaks at 420 and 530 °C detected on Cr/CeTi–C(x) catalysts. The one at low temperature (peak α, ∼420 °C) could be assigned to the reduction of CrO3 species to Cr2O3,37,38 and the other at high temperature (peak β, ∼530 °C) may be related to the reduction of CeO2 to Ce2O3.38,39 Moreover, compared to Cr/CeTi–C(0), the second reduction peak (β) shifts to lower temperature region and the amount of CeO2 species significantly increases. These results prove the fact that nonmetal-doping makes the reduction reaction taking place easier.3.4 Adsorption properties: O2-TPD
The adsorption property is an essential aspect to appraise the performance of the catalysts. O2-TPD was used to test the mobility of oxygen species,40 and the profiles of all Cr/CeTi–C(x) samples are shown in Fig. 4. It is widely reported that the enhanced mobility promotes the O2 adsorption and activation, which is the key step of NO oxidation.41,42 From Fig. 4, it is worth noting that there are two desorption peaks in the temperature range of 100–550 °C. The former at about 350 °C is ascribed to the chemical adsorption oxygen, and the latter between 350 °C and 750 °C is considered as the partial crystal oxygen.41 The different TPD profiles reveal the nonmetal doping indeed impacts the O2 adsorption. For the Cr/CeTi–C(1.2) catalyst, larger desorption band and lower desorption temperature indicate that the O2 adsorption capacity of the Cr/CeTi–C(1.2) catalyst is much higher than others. It is inferred that the oxygen atoms after doping are easy to migrate and generate oxygen vacancies.32 Thus, the nonmetal doping significantly promotes the mobility of oxygen.To further explore the reaction site, the catalytic activities with different sites were tested and their result is shown in Fig. S2a.† Compare to CeTi–C(1.2), CrOx, as the active site, plays the dominant role in the NO oxidation, and the material containing ceria plays a supporting role. In order to investigate their adsorption property, the TPD profiles (NO-TPD and O2-TPD) of CrOx, CeTi–C(1.2) and Cr/CeTi–C(1.2) are also displayed in Fig. S2b and c.† All samples possess the weak adsorption ability. However, it is obviously seen that CrOx is beneficial to adsorb and activate NO and CeTi–C(1.2) is inclined to activate O2. Moreover, there is a synergistic effect between CrOx and CeTi–C(1.2) on the Cr/CeTi–C(1.2) catalyst for the NO oxidation.
3.5 Chemical states analysis: XPS
To investigate the valence states of various species and verify the doping element, all catalysts were analyzed by XPS spectroscopy, which were presented in Fig. 5 and Table 1. | ||
| Fig. 5 High resolution XPS spectra of four Cr/CeTi–C(x) catalysts: (a) Cr 2p; (b) N 1s; (c) Ce 3d; (d) Ti 2p; and (e) O 1s. | ||
| Samples | Binding energy (eV) | Relative abundance (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| O | N | Cr6+/Crtot | Ce3+/Cetot | Oα/(Oα + Oβ) | ||||
| Oα | Oβ | Noxi | N–M | Npyr | ||||
| Cr/CeTi–C(0) | 530.7 | 529.3 | 403.7 | — | 396.7 | 45 | 22 | 33 |
| Cr/CeTi–C(0.6) | 530.5 | 529.1 | 403.7 | 399.3 | 396.7 | 47 | 27 | 36 |
| Cr/CeTi–C(1.2) | 530.5 | 529.1 | 403.5 | 399.3 | 396.5 | 61 | 45 | 41 |
| Cr/CeTi–C(1.8) | 530.5 | 529.1 | 403.5 | 399.3 | 396.5 | 47 | 31 | 37 |
The Cr 2p spectra of all the samples were numerically fitted with four components representing Cr2O3 species and CrO3 species, which is exhibited in Fig. 5a. The bands at about 586.9 and 577.0 eV are assigned to 2p1/2 and 2p3/2 of Cr2O3 species, while the peaks at about 588.1 and 578.2 eV are attributed to 2p1/2 and 2p3/2 of CrO3 species, respectively.39,43 XPS results verify the coexistence of two kinds of CrOx species (Cr2O3 and CrO3) on the surface of all samples. CrO3 is regarded as the active sites, and it exhibits the remarkable oxidability, which plays a vital role in the process of catalytic oxidation reaction.13,44 XPS elements and their surface concentrations are showed in Table 1. It is clearly seen that the high valence state CrO3 is generated via the suitable nonmetal doping. Meanwhile, the sequence of the ratio of Cr6+/Crtot follows as Cr/CeTi–C(1.2) > Cr/CeTi–C(0.6) > Cr/CeTi–C(1.8) > Cr/CeTi–C(0), which is in accordance with the catalytic activity in Fig. 1. Furthermore, it is worth noting that no-shift in binding energy is observed, which is due to the same density of electron cloud around Cr atoms. The results indicate that the doped site of nonmetal is not around the Cr atom.
According to the literature, the XPS spectra of N 1s (Fig. 5b) could be deconvoluted into two components at around 397 ± 1.0 and 402.7 ± 1.0 eV, and these peaks could be attributed to the pyridinic N45 and the oxidized nitrogen species,46 respectively. Particularly, for Cr/CeTi–C(1.2), a new weak band associated with N–metal compounds appears at 399.4 ± 0.5 eV is observed, which is attributed to the nitrogen species grafted on the metal.47 After the addition of cyanamide into the precursor, the active N–metal bond is detected, demonstrating that nonmetal is doped into the ceria–titanium solid solution and then formed the N–Ce and/or N–Ti bond(s). Furthermore, the actual amount of N–metal is not proportional to the addition of cyanamide, suggesting that there is an appropriate threshold value for the nonmetal doping. However, there is no change observed in the C 1s peak, as shown in Fig. S3,† which excludes the possibility of carbon doping. Based on above analysis, it comes to the conclusion that the doped nonmetal is N.
The valence states of the Ce species were also analyzed by fitting the curves of Ce 3d core level spectra obtained from XPS measurements, which is shown in Fig. 5c. Two groups of spin–orbital multiplets, corresponding to 3d3/2 and 3d5/2, are denoted as u and v and extend in the binding energy range of 872–924 eV.48 According to the literatures, the bands labeled v′ and u′ represent the 3d104f1 initial electronic state corresponding to Ce3+ ions, while the peaks marked v, v′′, v′′′ and u. u′′, u′′′ represent the 3d104f0 state of Ce4+ ions.49,50 Obviously, the chemical valence of Ce on the surface of Cr/CeTi–C(x) is mainly in a +4 oxidation state, and a small quantity of Ce3+ co-exists. It can be clearly seen from Table 1 that the addition of cyanamide displays the remarkable influence on the relative intensity of u′ and v′, indicating an increase of the surface Ce3+ content. The Ce3+ content of Cr/CeTi–C(1.2) (45%) is higher than that of Cr/CeTi–C(0) (22%). This might be attributed to the nonmetal doping, which could enhance their interaction between Ce and Ti to increase the amount of Ce3+.26 Usually, Ce3+ is accompanied by the existence of oxygen vacancies.51 Thus, more oxygen vacancies in Cr/CeTi–C(1.2) are favorable to improve the mobility of active oxygen species and enhance the redox property. Moreover, the Ce 3d binding energy of Cr/CeTi–C(1.2) sample shifts to higher energy compared to that of Cr/CeTi–C(0). The positive shift in binding energy is due to the difference of electronegativity of Ce (1.12 Pauling electronegativity scale) and N (3.04 Pauling electronegativity scale).26 Therefore, it further confirmed that nitrogen is successfully incorporated into the CeO2 lattice and substituted for oxygen around cerium.
As shown in Fig. 5d, the peaks of the samples appearing at around 458.7 and 464.5 eV are attributed to Ti 2p3/2 and Ti 2p1/2 of Ti4+.24 In contrast to Cr/CeTi–C(0), there is a slight positive shift of the Ti 2p binding energy in Cr/CeTi–C(1.2). This might be due to the lower density of electron cloud around Ti atoms than that in undoped catalyst, which further indicates that the nonmetal N with higher electronegativity (3.04 Pauling electronegativity scale) is also introduced into the Ti (1.54 Pauling electronegativity scale).26,52 However, the extent of positive shift in binding energy is different. The positive shift in ceria is stronger than that in titania. Therefore, it further confirms that nitrogen is mainly incorporated into the CeO2 lattice and partly introduced into the TiO2 lattice to substitute for oxygen.
As shown in Fig. 5e, the high-resolution O 1s spectra are mainly composed of two distinct peaks. It proves the existence of two types of oxygen species, i.e. lattice oxygen (denoted as Oβ) whose binding energy is 529.9 eV, and chemisorbed oxygen (denoted as Oα) whose binding energy is 531.2 eV.53 Based on the peak area integral of O 1s photoemissions, the relative concentration ratio of Oα/(Oα + Oβ) is calculated and shown in Table 1. It can be found that Oα/(Oα + Oβ) of Cr/CeTi–C(1.2) is higher than that of Cr/CeTi–C(0), which is 41% and 33%, respectively. This indicates that the nonmetal doping is conducive to generate more chemisorbed oxygen species. The chemisorbed oxygen is considered to be more active than the lattice oxygen. Some researchers23,54 have confirmed that the chemisorbed oxygen is the most active oxygen and benefits to activate O2 and then oxidize NO to NO2. This is one of the important reasons accounting for the promotional effect. Furthermore, it has been reported that the radius of N ion (0.171 nm) is close to that of O ion (0.140 nm), hence N ion is inclined to substitute oxygen position.52 This may support the hypothesis that the substitute N atoms occupy oxygen sites.
In a word, the presence of new N 1s peak is expected to confirm the nitrogen doping directly, whereas shifts in the Ce 3d, Ti 2p and O 1s binding energies verify the doping sites indirectly. Nonmetal doping is conducive to form oxygen vacancies and further generate active species, which helps NO oxidation. However, it is still vague about the doping sites. Herein, some other methods are used to identify the doping sites of nitrogen species.
3.6 Confirmation of doping sites: FTIR and Raman analysis
The gas–solid reactions usually take place on the surface of the catalysts. Thus, the confirmation of the doping sites is very important. To clarify this point, the samples of N–CeO2, N–TiO2 and N–CeTi were prepared and the corresponding FTIR spectra are displayed in Fig. 6a. It can be seen N–CeTi sample shows three main adsorption bands at 2970–2760, 1200–800 and 720–400 cm−1, which can be attributed to the vibration contribution(s) of N–metal bond,55 the bending vibration of the substituted –OH in CeOH35,36 and the vibrational mode of Ce–N–Ti structure,34 respectively. Furthermore, several obvious peaks at 1380, 1100–1000 and 668 cm−1 are clearly observed in the N–CeO2 and N–CeTi, which are not observed in N–TiO2. It is concluded that N species prefer to bond around cerium site rather than around titanium site. Bearing the above issues in mind, we could conclude that more N species are doped on the Ce–O–Ti and Ce–O–Ce sites and less N species affect the Ti–O–Ti structure. | ||
| Fig. 6 Fourier transform infrared (FTIR) spectroscopy (a) and visible-Raman spectra (b) of N–CeO2, N–TiO2 and N–CeTi samples. | ||
For a further study, Raman spectra were collected as the complementation of FTIR. As shown in Fig. 6b, it is well established that the first-order Raman spectrum of N–CeO2 exhibits a main band near 463 cm−1, which corresponds to the vibrational mode of the F2g symmetry in a cubic fluorite lattice.17 Meanwhile, the four peaks that are assigned to anatase TiO2 crystalline phase, observed at 144 (Eg), 397 (B1g), 516 (B1g), and 640 (Eg) cm−1, can be clearly distinguished in N–TiO2.54 However, N–CeTi, with a slight shift, exhibits much weaker and broader peaks than N–CeO2 and N–TiO2, indicating that N-doping might enhance the interaction between titanium and cerium.26 A small Raman peak located around 148 and 644 cm−1 is observed and consistent with a trace amount of anatase TiO2 (space group I41/amd, which has a very strong peak around this wavenumber), and a strong Raman peak at 463 cm−1 is assigned to CeO2 (space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), though it seem to be a weak band.56 Considering the tremendous difference of signal value of metal oxides, these results further demonstrate that N species prefer to doping around the cerium rather than doping around titanium. Moreover, two new bands at 194 and 377 cm−1 could be ascribed to N doped Ce–O–Ti bond and another peak at ca. 577 cm−1 can be associated with oxygen vacancies in the cerium–titanium solid solutions.56 It not only demonstrates the N doping is effective, but also proves that oxygen vacancies derive from Ce species. For this reason, it can be concluded that N species are inclined to substitute the O atoms of cerium oxides to form the Ce–N–Ti and Ce–N–Ce bonds.
m), though it seem to be a weak band.56 Considering the tremendous difference of signal value of metal oxides, these results further demonstrate that N species prefer to doping around the cerium rather than doping around titanium. Moreover, two new bands at 194 and 377 cm−1 could be ascribed to N doped Ce–O–Ti bond and another peak at ca. 577 cm−1 can be associated with oxygen vacancies in the cerium–titanium solid solutions.56 It not only demonstrates the N doping is effective, but also proves that oxygen vacancies derive from Ce species. For this reason, it can be concluded that N species are inclined to substitute the O atoms of cerium oxides to form the Ce–N–Ti and Ce–N–Ce bonds.
3.7 Confirmation of superoxide radicals: EPR analysis
Above conclusions have confirmed the dropping of N and the generated oxygen vacancies, what's more, considering the fact that oxygen vacancies can be activated to generate superoxide radicals, which are the important intermediates in the NO oxidation, it is necessary to verify the formation of superoxide radicals after the nonmetal doping. Two representative catalysts are analyzed by EPR spectroscopy to support this hypothesis.Fig. 7 displays the EPR spectra of superoxide radicals over the Cr/CeTi–C(0) and Cr/CeTi–C(1.2) catalysts. The theoretical EPR signals can be calculated using the effective spin Hamiltonian formula.57 Accordingly, the signal at g = 2.0061–2.0103 is characteristic of paramagnetic materials containing superoxide radicals58 and we detected the signal at g = 2.0076 in this study. The stronger intensity and larger peak area demonstrate that more superoxide radicals are generated in Cr/CeTi–C(1.2) than that in Cr/CeTi–C(0), which implies more superoxide radicals are produced after nonmetal doping. Such observations are consistent with the catalytic performance. Considering the fact that the oxygen vacancies in the metal oxide are the centers of the positive charges, thereby, O2 bounded electrons could easily generate the superoxide radicals (O2−) in the presence of oxygen vacancies, which are proven to be the important intermediates for NO oxidation. In other words, it means that N doping could improve the formation of superoxide radicals.
3.8 Analysis of surface species: in situ DRIFTS
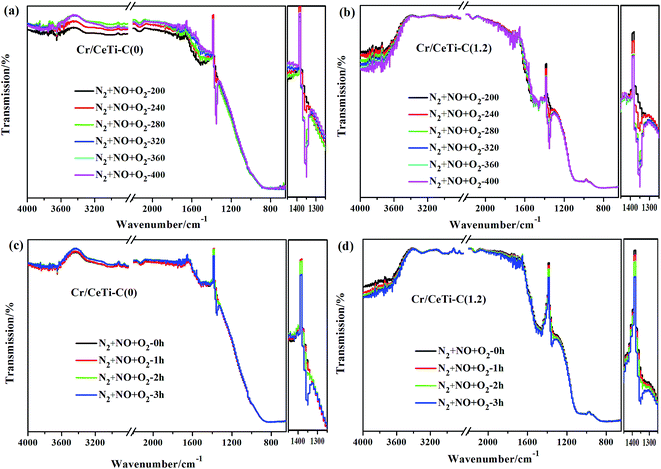
In order to investigate the nature of surface-bound species and provide the valuable insight into the mechanism of heterogeneous catalytic reactions, especially under in situ conditions, in situ DRIFTS spectra of different catalysts were recorded at different temperatures with time. Fig. 8a–b illustrates the evolution of the spectra recorded on Cr/CeTi–C(0) and Cr/CeTi–C(1.2) catalysts at different temperatures. The experiment temperatures are set to simulate the test temperature as far as possible. A noticeable peak, both observed in Cr/CeTi–C(0) and Cr/CeTi–C(1.2), is detected at 1352 cm−1, which gradually increases in intensity with the increase of temperature. It is in accordance with the literature41,59 and ascribed to the adsorbed nitrate, which is generated by the interaction between chemisorbed NO and superoxide radicals (O2−). The stable temperature of Cr/CeTi–C(1.2) (280 °C) is lower than that of Cr/CeTi–C(0) (320 °C), which suggests the increased superoxide radical (O2−) is formed after the cyanamide addition. It is consistent with the results of catalytic performance and EPR analysis. In the –OH stretching region, a broad absorption peak between 3600 and 3000 cm−1 is obviously distinct, which is ascribed to the stretching vibrations of surface hydroxyl (–OH) species.16 Additionally, a set of bands in the range 1750–1450 cm−1 is also observed on two representative catalysts. The bands from 1500 to 1460 cm−1 corresponds to the chelating nitro(–NO2),60 while the band at 1712 cm−1 is assigned to the free nitrate.61 These results implies that both nitro(–NO2) and nitrates are intermediates in NO oxidation. Meanwhile, combined with the band at 1352 cm−1 coordinated to adsorbed nitrate, the Cr/CeTi–C(1.2), exhibits a new nitrate band, indicating that nonmetal doping is beneficial to generate the superoxide radicals and further facilitate the formation of nitrate, which support our hypothesis. However, a significant band associated with nitrosyl appeared at 2132 cm−1 is observed, regardless of the presence of dopant, which is proven to be the reaction intermediate in NO oxidation.62,63 Especially, the bands ranged from 1100 to 800 cm−1 appear after the cyanamide doping, which are assigned to the Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching.64 This result suggests that the chromium oxide with high valence state is stable and abundant, which plays a crucial role in the adsorption and activation of NO. This observation is consistent with the activity results.
O stretching.64 This result suggests that the chromium oxide with high valence state is stable and abundant, which plays a crucial role in the adsorption and activation of NO. This observation is consistent with the activity results.
In situ DRIFTS spectra of interaction between NO and O2 as a function of time are displayed in Fig. 8c and d for Cr/CeTi–C(0) and Cr/CeTi–C(1.2) catalysts. Obviously, several strong bands at 3600–3200, 1500–1460, 1352 cm−1 are detected in the different reaction time at 320 °C as well as some weak bands appear at 2132 and 1712 cm−1. All the bands intensities increase with time going and stabilize at about 3 h. According to the literatures,10,21,43,60,61 the bands at 3600–3200 cm−1 are assigned to the surface hydroxyl (–OH) species, the band at 2132 cm−1 is attributed to the nitrosyl, the bands at 1712 and 1352 cm−1 are typical of free nitrate and adsorbed nitrate, and the other bands between 1500 and 1460 cm−1 are associated with the chelating nitro(–NO2). However, there are obvious differences on the details between two samples, that is, the intensities of adsorbed nitrate and chelating nitro(–NO2) of Cr/CeTi–C(1.2) catalyst is higher than that of Cr/CeTi–C(0) catalyst. More chemical oxygen are activated after the cyanamide addition, and further react with NO to generate chelating nitro(–NO2). Meanwhile, the formation of adsorbed nitrate could be attributed to the interaction of NO and superoxide radicals. These reasons support the notion that the suitable N doping is conducive to accelerate the process of NO oxidation.
3.9 Possible catalytic mechanism of NO oxidation
Based on the above-mentioned observations of activity test and characterization results, a possible catalytic mechanism of NO oxidation is tentatively proposed to further understand the promotional effect of the nonmetal doping. It is illustrated in Fig. 9 and described as follows: CrO3, as the main active sites, could preferentially oxidize NO to form intermediate nitrosyl (NO+), which further reacts with O2 to form nitrate species. Moreover, N element is successfully doped into the ceria–titanium solid solution and then enhance their properties, and thereby, the addition of cyanamide is conductive to tune O2 activation and improve NO adsorption strength, which are caused by two reasons. On one hand, more chemical oxygen are activated, and further react with NO to generate chelating nitro(–NO2). On the other hand, N element is inclined to substitute the O atom around cerium to form the Ce–N–Ti and Ce–N–Ce bonds, which is accompanied by oxygen vacancies. Oxygen vacancies could be activated by O2 to form superoxide radicals. The interaction of NO and superoxide radicals leads to the formation of adsorbed nitrate. Finally, the intermediates NO+ and nitrates interact quickly to generate gaseous NO2.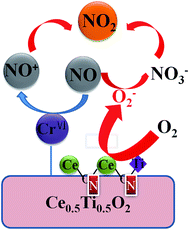 | ||
| Fig. 9 Possible reaction model of selective catalytic oxidation of NO over the Cr/CeTi–C(x) catalysts. | ||
4 Conclusions
In summary, the promotional effect and mechanism study of nonmetal-doped Cr/CexTi1−xO2 were investigated for NO oxidation. The Cr/CeTi–C(1.2) catalyst exhibited the excellent catalytic activity and selectivity for NO oxidation, with 61.5% conversion and 60% selectivity at 320 °C, respectively. Combined with XRD, FTIR, H2-TPR, O2-TPD, XPS, Raman, EPR and in situ DRIFTS results, it is demonstrated that the addition of cyanamide generates the superoxide radicals, which is conductive to tune O2 activation and NO adsorption strength simultaneously to improve the catalytic performance. Nevertheless, the doping element and sites are also taken into account. That is, N element is inclined to substitute the O atoms around cerium to form the Ce–N–Ti and Ce–N–Ce bonds, which is beneficial to generate superoxide radicals. Furthermore, the interaction of NO and superoxide radicals leads to the formation of adsorbed nitrate, which is detected by the investigation of in situ DRIFTS. Finally, the intermediates NO+ caused by CrOx and nitrates interact quickly to generate gaseous NO2.Acknowledgements
This work is financially supported by the Assembly Foundation of The Industry and Information Ministry of the People's Republic of China 2012 (543), the National Natural Science Foundation of China (51408309 and 51578288), Science and Technology Support Program of Jiangsu Province (BE2014713), Natural Science Foundation of Jiangsu Province (BK20140777), Industry-Academia Cooperation Innovation Fund Projects of Jiangsu Province (BY2014004-10), Science and technology project of Nanjing (201306012), Jiangsu Province Scientific and Technological Achievements into a Special Fund Project (BA2015062), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education of Jiangsu Higher Education Institutions.References
- Z. Y. Sheng, Y. F. Hu, J. M. Xue, X. M. Wang and W. P. Liao, J. Rare Earths, 2012, 30, 676 CrossRef CAS.
- I. Spasova, P. Nikolov and D. Mehandjiev, J. Colloid Interface Sci., 2005, 290, 343 CrossRef CAS PubMed.
- M. F. Irfan, J. H. Goo and S. D. Kim, Appl. Catal., B, 2008, 78, 267 CrossRef CAS.
- M. P. Ruggeri, A. Grossale and I. Nova, Catal. Today, 2012, 184, 107 CrossRef CAS.
- N. Takahashi, K. Yamazaki and H. Sobukawa, Appl. Catal., B, 2007, 70, 198 CrossRef CAS.
- Z. Q. Liu, W. S. Epling and J. A. Anderson, J. Phys. Chem. C, 2011, 115, 952 CAS.
- G. Liu and P. X. Gao, Catal. Sci. Technol., 2011, 1, 552 CAS.
- Z. Ren, Y. B. Guo, Z. H. Zhang, C. H. Liu and P. X. Gao, J. Mater. Chem. A, 2013, 1, 9897 CAS.
- K. Li, X. Tang and H. H. Yi, Chem. Eng. J., 2012, 192, 99 CrossRef CAS.
- L. D. Li, Q. Shen, J. Cheng and Z. P. Hao, Appl. Catal., B, 2010, 93, 259 CrossRef CAS.
- D. Bhatia, R. W. McCabe, M. P. Harold and V. Balakotaiah, J. Catal., 2009, 266, 106 CrossRef CAS.
- Z. B. Wu, N. Tang, L. Xiao, Y. Liu and H. Q. Wang, J. Colloid Interface Sci., 2010, 352, 143 CrossRef CAS PubMed.
- W. Cai, Q. Zhong and W. Zhao, Chem. Eng. J., 2014, 246, 328 CrossRef CAS.
- H. Tong, J. Luo, Z. Q. Tong, B. Xia and H. Luo, Environ. Eng. Sci., 2011, 28, 711 CrossRef CAS.
- H. D. Liu, L. Q. Wei and R. L. Yue, Catal. Commun., 2010, 11, 829 CrossRef CAS.
- N. Tang, Y. Liu, H. Q. Wang and Z. B. Wu, J. Phys. Chem. C, 2011, 115, 8214 CAS.
- J. Guzman, S. Carrettin and A. Corma, J. Am. Chem. Soc., 2005, 127, 3286 CrossRef CAS PubMed.
- I. D. Gonzalez, R. M. Navarro, W. Wen, N. Marinkovic, J. A. Rodriguez, F. Rosa and J. L. G. Fierro, Catal. Today, 2010, 149, 372 CrossRef CAS.
- J. L. Ye, Y. Q. Wang, Y. Liua and H. Wang, Int. J. Hydrogen Energy, 2008, 33, 6602 CrossRef CAS.
- P. Li, Y. Xin, Q. Li, Z. P. Wang, Z. L. Zhang and L. R. Zheng, Environ. Sci. Technol., 2012, 46, 9600 CrossRef CAS PubMed.
- J. Ding, Q. Zhong and S. L. Zhang, Ind. Eng. Chem. Res., 2015, 54, 2012 CrossRef CAS.
- Y. T. Li and Q. Zhong, J. Hazard. Mater., 2009, 172, 635 CrossRef CAS PubMed.
- H. Y. Li, S. L. Zhang and Q. Zhong, J. Colloid Interface Sci., 2013, 402, 190 CrossRef CAS PubMed.
- W. Zhao, Q. Zhong, Y. X. Pan and R. Zhang, Chem. Eng. J., 2013, 228, 815 CrossRef CAS.
- F. Y. Wei, L. S. Ni and P. Cui, J. Hazard. Mater., 2008, 156, 135 CrossRef CAS PubMed.
- R. Zhang, Q. Zhong, W. Zhao, L. M. Yu and H. X. Qu, Appl. Surf. Sci., 2014, 289, 237 CrossRef CAS.
- E. M. Neville, M. J. Mattle, D. Loughrey, B. Rajesh and M. Rahman, J. Phys. Chem. C, 2012, 116, 16511 CAS.
- J. Virkutyte, B. Baruwatix and R. S. Varma, Nanoscale, 2010, 2, 1109 RSC.
- S. Zhou, Y. Liu, J. M. Li, Y. J. Wang, G. Y. Jiang and Z. Zhao, Appl. Catal., B, 2014, 158–159, 20 CrossRef CAS.
- B. Choudhury, B. Borah and A. Choudhury, J. Photochem. Photobiol., A, 2012, 88, 257 CrossRef CAS PubMed.
- S. Letichevsky, C. A. Tellez, R. R. D. Avillez, M. I. P. D. Silva and L. G. Appel, Appl. Catal., B, 2005, 58, 203 CrossRef CAS.
- X. J. Yao, Y. Xiong, W. X. Zou and L. Dong, Appl. Catal., B, 2014, 144, 152 CrossRef CAS.
- L. J. Liu, X. J. Yao, B. Liu and L. Dong, J. Catal., 2010, 27, 45 CrossRef.
- J. S. Zhang, J. H. Sun, K. Maeda, K. Domen and X. C. Wang, Energy Environ. Sci., 2011, 4, 675 CAS.
- K. Nakamoto, Handbook of Vibrational Spectroscopy, John Wiley & Sons, Ltd., 2006, p. 1872 Search PubMed.
- C. X. Gao, Q. F. Liu and D. S. Xue, J. Mater. Sci. Lett., 2002, 21, 1781 CrossRef CAS.
- R. Ma, P. Hu, L. Jin, Y. Wang, J. Lu and M. Luo, Catal. Today, 2011, 175, 598 CrossRef CAS.
- B. Grzybowska, J. Słoczynski, R. Grabowski, K. Wcislo and J. Zielinski, J. Catal., 1998, 178, 687 CrossRef CAS.
- P. Yang, Z. H. Meng, S. S. Yang, Z. N. Shi and R. X. Zhou, J. Mol. Catal. A: Chem., 2014, 393, 75 CrossRef CAS.
- Z. Q. Zou, M. Meng and Y. Q. Zha, J. Phys. Chem. C, 2011, 114, 468 Search PubMed.
- W. Cai, Q. Zhong and W. Zhao, Appl. Catal., B, 2014, 158–159, 258 CrossRef CAS.
- L. Zhong, W. Cai, Y. Yu and Q. Zhong, Appl. Surf. Sci., 2015, 325, 52 CrossRef CAS.
- Y. Wang, A. P. Jia, M. F. Luo and J. Q. Lu, Appl. Catal., B, 2015, 165, 477 CrossRef CAS.
- L. Zhong, Y. Yu, W. Cai, X. X. Geng and Q. Zhong, Phys. Chem. Chem. Phys., 2015, 17, 15036 RSC.
- X. Li, H. Wang, J. T. Robinson, H. Sanchez, G. Diankov and H. Dai, J. Am. Chem. Soc., 2009, 131, 15939 CrossRef CAS PubMed.
- D. Choudhury, B. Das, D. D. Sarma and C. N. R. Rao, Chem. Phys. Lett., 2010, 497, 66 CrossRef CAS.
- H. L. Peng, S. Y. Hou, D. Dang and B. Q. Zhang, Appl. Catal., B, 2014, 158–159, 60 CrossRef CAS.
- C. X. Liu, L. Chen, J. H. Li and L. Ma, Environ. Sci. Technol., 2012, 46, 6182 CrossRef CAS PubMed.
- X. Li, S. J. Wei, Z. L. Zhang, Y. X. Zhang and X. Y. Gao, Catal. Today, 2011, 175, 112 CrossRef CAS.
- L. Chen, J. H. Li and M. F. Ge, J. Phys. Chem. C, 2009, 113, 21177 CAS.
- J. Fan, X. Wu, X. Wu, Q. Liang and D. Weng, Appl. Catal., B, 2008, 81, 38 CrossRef CAS.
- T. Yu, X. Tan, L. Zhao, Y. X. Yin and J. Wei, Chem. Eng. J., 2010, 157, 86 CrossRef CAS.
- X. F. Tang, Y. G. Li and X. Huang, Appl. Catal., B, 2006, 62, 265 CrossRef CAS.
- R. Brosius and J. A. Martens, Top. Catal., 2004, 28, 119 CrossRef CAS.
- M. J. Munoz-Batista, M. Fernández-García and A. Kubacka, Appl. Catal., B, 2015, 164, 261 CrossRef CAS.
- L. G. Kong, D. J. Gregg, I. Karatchevtseva, S. C. Middleburgh and G. Triani, Inorg. Chem., 2014, 53, 6761 CrossRef CAS PubMed.
- J. Matta, D. Courcot, E. Abi-Aad and A. Aboukaïs, Chem. Mater., 2002, 14, 4118 CrossRef CAS.
- M. Anpo, M. Che, B. Fubini, E. Garrone and E. Giamello, Top. Catal., 1999, 8, 189 CrossRef CAS.
- J. A. Rodriguez, T. Jirsak, J. Dvorak, S. Sambasivan and D. Fischer, J. Phys. Chem. B, 2000, 104, 319 CrossRef CAS.
- P. Sazama, L. Capek, H. Drobná and Z. Sobalík, J. Catal., 2005, 232, 302 CrossRef CAS.
- T. J. Toops, D. Barton Smith and W. P. Partridge, Appl. Catal., B, 2005, 58, 245 CrossRef CAS.
- E. Ivanova, K. Hadjiivanov, D. Klissurski, M. Bevilacqua, T. Armaroli and G. Busca, Microporous Mesoporous Mater., 2001, 46, 299 CrossRef CAS.
- K. Hadjiivanov and H. Knozinger, Phys. Chem. Chem. Phys., 2000, 2, 2803 RSC.
- P. G. Harrison and W. Daniell, Chem. Mater., 2001, 13, 1708 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01928e |
| This journal is © The Royal Society of Chemistry 2016 |