Effect of ultrasonication on Ni–Mo coatings produced by DC electroformation
Abstract
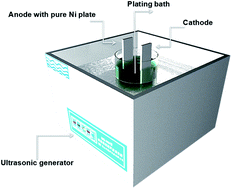
The effect of the ultrasound-assisted method on Ni–Mo alloy coatings by direct current (DC) electroformation in a sulphate–citrate bath was investigated with different ultrasound powers. This study focused on the microstructure, molybdenum content, grain size, roughness, micro-hardness and corrosion resistance of the Ni–Mo alloy coatings, which were influenced by the ultrasound parameters. X-ray diffraction results showed that ultrasound modification decreased the grain size of the nanocrystalline alloy coatings. The hardness of the as-plated coatings after ultrasonication was determined by Vickers diamond indentation tests and compared with that of the Ni–Mo coatings in the absence of ultrasound. As a result, the hardness (719 HV) of the former coatings improved significantly. Scanning electron microscopy and atomic force microscopy images showed a smoother coating and a decrease in the surface roughness of the electrodeposited film, as a result of the use of ultrasound. Furthermore, the corrosion resistance behaviour of the coatings was analysed by a Tafel polarisation curve and electrochemical impedance spectroscopic studies in a 3.5 wt% NaCl solution. The ultrasonicated coatings (Ni–Mo) significantly enhanced corrosion resistance (4.1 μA cm−2) compared with those without ultrasound assistance. However, at the maximum ultrasound power (270 W), the molybdenum content, grain size, roughness, microhardness and corrosion resistance of the Ni–Mo alloy coatings all slightly decreased.

 Please wait while we load your content...
Please wait while we load your content...