Sb doped Li4Ti5O12 hollow spheres with enhanced lithium storage capability
Fuyun Li,
Min Zeng*,
Jing Li,
Xiaoling Tong and
Hui Xu
School of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, PR China. E-mail: zengmin@swust.edu.cn
First published on 8th March 2016
Abstract
In this work, the Sb doped Li4Ti5O12 hollow spheres were synthesized by a combination of a hydrothermal method and a solid state reaction. The Sb doped Li4Ti5O12 hollow spheres were characterized by X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, transmission electron microscopy and high-resolution transmission electron microscopy. These indicated that Sb doped Li4Ti5O12 hollow spheres were synthesized successfully and Sb ions were uniformly dispersed into the Li4Ti5O12 lattice without changing the structure of Li4Ti5O12. The electrochemical measurements, including constant current charge–discharge and electrochemical impedance spectroscopy, demonstrated that the Sb-doped Li4Ti5O12 hollow spheres showed an excellent reversible capacity and remarkable cycling performance, even under conditions of high current densities.
Introduction
Lithium-ion batteries (LIBs) have achieved commercial success in the field of portable electronic devices owing to their outstanding properties, such as high energy density, no memory effect, light weight, long lifespan, and environmental benignity, especially as an important energy storage device for electric vehicles (EVs) and hybrid electric vehicles (HEVs).1–5 Commercial applications of batteries are based upon graphite anode material, but the carbon-based materials as anode materials suffer greatly from effects associated with severe safety hazards and poor cycle life.6–9 This has accelerated a myriad of investigations that have been hitherto conducted aiming at the development of new electrode materials having improved electrochemical and safety performances. Spinel Li4Ti5O12 has been regarded as one of the most promising alternatives for graphite based on its excellent cyclic reversibility (a consequence of almost zero structural volume change during Li ion insertion/extraction) and high safety.10–12 However, the inherently kinetic problems, that is, low electrical conductivity (ca. 10−13 S cm−1), limit its rate capability.13–15 The most commonly used ways to solve this problem are to reduce the particle size,16–18 to dope with conductive non-metal/metal ions (e.g., F−, Na+, Zn2+, La3+, Zr4+, V5+, Nb5+, Mo4+)19–26 and to coat conductive materials (e.g., CeO2, grapheme, carbon, N-doped carbon) on the Li4Ti5O12 surface.5,27–29 Li4Ti5O12 nanomaterials with high surface area can significantly improve the rate capability. However, the large surface area also gives a low tap density, which will reduce the volumetric energy density. Doping with conductive non-metal/metal ions in materials can lead to an increased electrical conductivity and a better lithium-ion insertion/extraction performance.19–26 Coating conductive materials on the Li4Ti5O12 surface enhances the surface conductivity and the electrical contact in the electrode; therefore the method can improve the rate capability of electrode materials. Nonetheless, most of the processes are either complex or have to be performed at high temperature (>600 °C). In addition, inspired by the successful use of many other metal oxide-based hollow structures in LIBs,30 Li4Ti5O12 hollow structures with porous shells exhibit many structural advantages. Specifically, the nanosized primary particles facilitate Li+ ion transport by dramatically reducing the lithium diffusion length. Moreover, the hollow structures with high surface area allow good penetration of the electrolyte into the active materials. Furthermore, the robust framework of the secondary hollow structures prevents the aggregation of particles. As a result, when evaluated as anode materials for LIBs, the Li4Ti5O12 hollow spheres with doping with conductive metal ions manifest excellent long cycle stability.Based on the merits mentioned above, we reported a practical and effective method to synthesize the Sb-doped Li4Ti5O12 hollow spheres through a two-step process. The advantages of using doping with Sb5+ to improve the Li4Ti5O12 hollow spheres in LIBs applications are investigated in this paper. The results demonstrated that the Sb-doped Li4Ti5O12 hollow spheres showed an excellent reversible capacity and outstanding cycling performance, even under conditions of high current densities.
Experimental
Preparation of Sb-doped Li4Ti5O12 hollow spheres with anode material
Materials characterizations
The samples were initially measured by X-ray diffraction using an X-ray diffraction analyzer with Cu-Kα radiation (X'Pert PRO, PANalytical). The surface chemical composition and the oxidation valence states of both samples were measured on an X-ray photoelectron spectroscopy (XPS), using a Perkin-Elmer model PHI 5600 XPS system. The morphologies and doped element distribution of Sb-LTO were observed using a field emission scanning electron microscope (Ultr55, Zeiss). The more detailed structural information was measured on a transmission electron microscope (TEM) and a high-resolution transmission electron microscope (HRTEM, Tecnai F20, FEI).Electrochemical measurements
The working electrodes were prepared by mixing the as-prepared materials, super P and polyvinylidene difluoride (PVDF) with an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 weight ratio. The uniform slurry was coated on a Cu foil and dried at 120 °C for 24 h to remove the solvent. The coil cells CR2016 were assembled with pure lithium foil as the counter electrode, a polypropylene microporous films as separator, and 1 M LiPF6 EC
10 weight ratio. The uniform slurry was coated on a Cu foil and dried at 120 °C for 24 h to remove the solvent. The coil cells CR2016 were assembled with pure lithium foil as the counter electrode, a polypropylene microporous films as separator, and 1 M LiPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1
DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrolyte in an argon-filled glove box. The charge–discharge measurement at room temperature was carried out on a LAND test system with cut-off voltage of 1.0–2.5 V (vs. Li/Li+) at various constant current densities (1–10C). Electrochemical impedance spectroscopy (EIS) was measured by means of a CHI760C electrochemical station in the frequency range of 0.01 Hz to 10 kHz.
1) electrolyte in an argon-filled glove box. The charge–discharge measurement at room temperature was carried out on a LAND test system with cut-off voltage of 1.0–2.5 V (vs. Li/Li+) at various constant current densities (1–10C). Electrochemical impedance spectroscopy (EIS) was measured by means of a CHI760C electrochemical station in the frequency range of 0.01 Hz to 10 kHz.
Results and discussion
Fig. 1 shows the XRD patterns of pure TiO2 and Sb doped TiO2, and the main diffraction patterns of both samples can be well indexed based on a anatase titanium dioxide, implying that the Sb doping does not change the structure of TiO2. The crystallite size of TiO2 and Sb doped TiO2 calculated using the Scherrer equation is about 13.5 and 12.5 nm, respectively, indicating that TiO2 and Sb doped TiO2 hollow spheres are composed of nanocrystalline subunits.The morphologies and microstructure of the pure TiO2 and original Sb doped TiO2 hollow spheres are examined by scanning electron microscopy (SEM) (Fig. 2). The result implies that the pure TiO2 and Sb doped TiO2 hollow spheres with an average diameter of around 3 μm were synthesized successfully via a general one-pot template-free hydrothermal strategy. The pure TiO2 and Sb doped TiO2 hollow spheres are composed of nanocrystalline subunits of around 15 nm, which is further confirmed by the XRD calculation using the Scherrer equation. In addition, the EDX mapping images of Ti, O and Sb elements are shown in Fig. 3. The distribution of Sb element is consistent with that of Ti and O, which indicates that Sb is uniformly distributed in the TiO2 crystal structure by means of a simple hydrothermal method. Based on the discussion mentioned above, we successfully synthesized the pure TiO2 and Sb doped TiO2 hollow spheres.
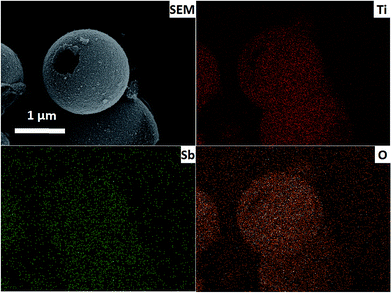 | ||
| Fig. 3 SEM image of Sb doped TiO2 hollow spheres and the EDX mapping images of Ti, Sb and O elements. | ||
The XRD patterns of both Sb-LTO and LTO are consistent with JCPDS card no. 49-0207 (Fig. 4a), and all peaks can be indexed to the spinel phase with the space group Fd3m. This result indicates that there is no effect on the crystal structure of spinel LTO with the addition of antimony pentoxide. The mean crystallite size of Sb-LTO and LTO calculated from Scherrer's formula is 169.2 nm and 128.1 nm, respectively, indicating that the particle size of nanoparticles of LTO and Sb-LTO obtained after annealing at 800 °C are much larger than the TiO2 and Sb doped TiO2 precursor spheres. In addition, the ionic radius of Sb5+ (0.6 Å) was nearly the same as that of Ti4+ (0.605 Å), and hence Sb ions were easily incorporated into the Ti sites. No secondary peaks belonging to Sb2O5 were detected in the XRD patterns of Sb-LTO in Fig. 4a, which indicated that the doped Sb ions might be introduced into Ti sites in the LTO lattice without changing its structural characteristics.
 | ||
| Fig. 4 (a) XRD patterns and (b) XPS survey spectra of LTO and Sb-LTO hollow spheres. Ti 2p XPS spectra of (c) LTO and (d) Sb-LTO. | ||
Fig. 4b illustrates the XPS survey spectra of LTO and Sb-LTO. A new peak at around 773.6 eV for Sb-LTO can be seen clearly, which can be ascribed to Sb 3p, implying the presence of Sb in the surface of the doped specimen. The high-resolution XPS spectra of Ti 2p for LTO and Sb-LTO are shown in Fig. 4c and d. For LTO, two broad peaks at ∼464.4 and ∼458.6 eV correspond well with characteristic Ti 2p1/2 and Ti 2p3/2 peaks of Ti4+, and it also had two tiny peaks near 457.0 and 463.8 eV, which were attributed to Ti3+. However, there has been a decrease in the binding energy of Ti 2p in Sb-LTO when compared to LTO. This indicates that treatment with Sb5+ doping changed the surface bonding of LTO. It can be seen that the contents of Ti3+ at 2p3/2 and 2p1/2 in Fig. 4d are 10.41% and 27.57%, respectively, which are much higher than the content in LTO (Fig. 4c). This implies that Ti4+ may be partially reduced to Ti3+. On the basis of the results of Rietveld refinement and XPS analysis, Sb5+ must have been doped into Ti sites of LTO. This result is consistent with the XRD results (Fig. 4a). The above discussions indicate that Sb5+ doping into Ti sites can promote the reduction of Ti4+ to Ti3+ to balance the charge. It was reported that the Ti3+ ions in LTO can effectively improve the concentration of electron–hole;23 therefore, a larger proportion of Ti3+ can effectively improve bulk electrical conductivity. In consequence, Sb-LTO would exhibit preferable electrical conductivity.
Meanwhile, as revealed in Fig. 5a–d, the morphologies and structures of both samples were investigated by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM). The corresponding SEM images of as-prepared LTO (Fig. 5a and b) and Sb-LTO (Fig. 5c and d) show that LTO and Sb-LTO hollow spheres with an average diameter of around 3.5 μm were synthesized successfully via solid-state reaction method. The result implies that the second solid-state reaction of Li2CO3 with Sb-doped TiO2 can still preserve the hollow structures even after mechanical mixing and high temperature sintering. Benefiting from the relatively mild reaction conditions, the shell structure can withstand the mechanical mixing and high temperature sintering without collapse or deformation. The LTO and Sb-LTO hollow spheres are composed of nanoparticles with a particle size of around 150 nm. The results indicated that the sphere diameters and particle size of nanoparticles of LTO and Sb-LTO hollow spheres are larger than the TiO2 and Sb doped TiO2 precursor spheres. Moreover, the TEM image in Fig. 5e shows that the Sb-LTO is hollow structures, which is consistent with the results of SEM. The Sb-LTO has a well-defined crystalline structure, and the (111) facet of the Sb-LTO is ca. 4.8 Å (Fig. 5f), which is a bit smaller than the pure LTO. This can be explained by substitution of Sb5+ for Ti4+. The ionic radius of Sb5+ and Ti4+ is similar, but the electrovalence of Sb5+ is higher than that of Ti4+. Thus, the ionic bonding interactions between Sb5+ and neighboring atoms are stronger than that between Ti4+ and neighboring atoms, which decreases the interplanar spacing.
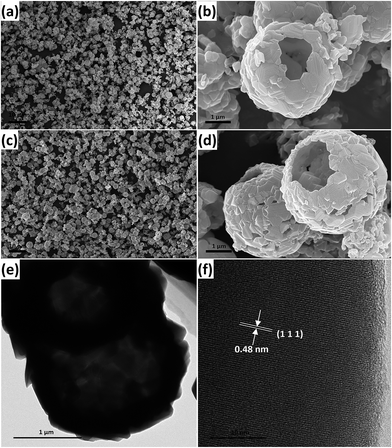 | ||
| Fig. 5 SEM images of (a and b) LTO and (c and d) Sb-LTO. (e) TEM and (f) HRTEM images of the as-synthesized Sb-LTO. | ||
The Sb-LTO hollow spheres was further analyzed using EDX mapping (Fig. 6), which implies that all of the Ti, O and Sb were distributed homogeneously within the particles of Sb-LTO hollow spheres.
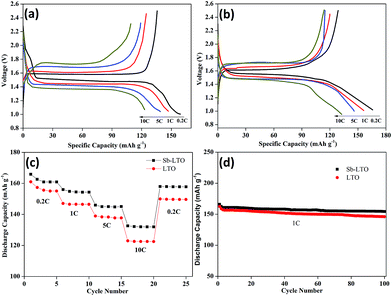
The electrochemical performances of pure LTO and Sb-LTO hollow spheres was evaluated in order to study the effects of Sb5+ doping systematically (Fig. 7). The initial charge–discharge curves of pure LTO and Sb-LTO was evaluated in the range of 1–2.5 V at different charge–discharge rates from 0.2 to 10C (Fig. 7a and b). As demonstrated in the charge and discharge profiles, the electrochemical performances of the LTO are markedly improved through Sb5+ doping. The pure LTO electrodes can display the electrochemical performances of the LTO are markedly improved through Sb5+ doping. The pure LTO electrodes can display the first specific capacities of 160.1 mA h g−1, 146.99 mA h g−1, 138.96 mA h g−1 and 123 mA h g−1 at the rates of 0.2C, 1C, 5C and 10C, respectively, while Sb-LTO can deliver the first specific capacities of 165.8 mA h g−1, 156.2 mA h g−1, 146 mA h g−1 and 132.7 mA h g−1 at the same rates. It is found that the discharge capacities for both electrodes decrease with increase of current densities. However, it is important to note that the discharge capacities of LTO electrodes decrease more rapidly than the Sb-LTO electrodes with increase of current densities. Therefore, the Sb-LTO electrodes showed much improved rate retention capability compared with the pure LTO electrodes. It is generally known that the potential difference (DE) between charge plateau and discharge plateau can reflect the polarization degree of the electrodes. The potential difference of the Sb-LTO electrodes is much lower than that of the pure LTO electrodes at different rates, which means that the former has less polarization and better reversibility than pure LTO electrodes.
The rate performance of the LTO and Sb-LTO electrodes is shown in Fig. 7c. Although the initial several cycles have slight fading of capacity at 0.2C, galvanostatic measurements (Fig. 7c) indicate that the Sb-LTO hollow spheres can be reversibly cycled at 1, 5 and 10C with remarkable specific capacities of 156.1, 146 and 132.7 mA h g−1, respectively. When the current rate is finally reduced back to 0.2C, the capacity returns to almost the original value of 165.9 mA h g−1, which again suggests that the Sb-LTO have good reversibility. The reversibility and special discharge capacities of Sb-LTO are superior to the pure LTO, which agrees with the initial charge–discharge curves of pure LTO and Sb-LTO at various rates.
This clearly demonstrates that the Sb-LTO is greatly beneficial to the rate performance at various charge and discharge currents. The superior rate performance for Sb-LTO can be attributed to the synergetic effects of Sb5+ doping and the presence of hollow spheres in which Sb5+ doping has played a critical role in improving the electrochemical performance.
The cycle performance of LTO and Sb-LTO has been studied for 100 cycles at the rate of 1C, as shown in Fig. 7d. It can be observed that Sb-LTO presents better cycling stability than pure LTO at 1C rate. In particular, after 100 cycles, the Sb-LTO can maintain a specific capacity of 154.4 mA h g−1 with a capacity loss of 6.9% at 1C/1C rate, and the LTO only maintained 146 mA h g−1 with a capacity loss of 10.2% at the same rate. This result demonstrates that the Sb5+ doping can improve the cycling stability of LTO anode materials. Mechanism for the enhanced electrochemical performance of LTO was attributed to that the doped Sb ions might be introduced into Ti sites in the LTO lattice, which can promote the reduction of Ti4+ to Ti3+ to balance the charge. It was reported that the Ti3+ ions in LTO can effectively improve bulk electrical conductivity,31 which contributes to the superior electrochemical performance for Sb-LTO.
To further gain insight into the reason of improved performance of Sb-LTO, electrochemical impedance spectroscopy (EIS) measurement of the LTO and Sb-LTO electrodes was performed, and typical Nyquist plots were fitted by a simple modified Randles–Ershler equivalent circuit,32 as shown in Fig. 8. The impedance curves show one compressed semicircle in the medium-frequency region, which could be considered to charge-transfer resistance (Rct) at the particle/electrolyte interface, and an inclined line in the low-frequency range, which could be assigned to be a Warburg impedance (Zw). Moreover, Rs is the resistance of electrolyte. The values of Rs, Rct and Zw are obtained from the simulated data of EIS by the equivalent circuit, listed in Table 1. The Rs, Rct and Zw of the Sb-LTO are 4.11 Ω, 25.99 Ω and 20.54 Ω, respectively, which are smaller than those of pure LTO. The diffusion coefficient of lithium-ion was estimated to be 3.27 × 10−13 cm2 s−1 and 1.28 × 10−12 cm2 s−1 for LTO and Sb-LTO, respectively. This experiment demonstrated that Sb5+ doping can effectively restrain the charge-transfer resistance of LTO electrodes and enhanced the Li+ diffusion.
 | ||
| Fig. 8 The electrochemical impedance spectra of LTO and Sb-LTO hollow spheres after the first three times cycle. | ||
| Samples | Rs | Rct | Zw | DLi+ (cm2 s−1) |
|---|---|---|---|---|
| Pure LTO | 4.44 | 42.56 | 32.96 | 3.27 × 10−13 |
| Sb-LTO | 4.11 | 25.99 | 20.54 | 1.28 × 10−12 |
Conclusions
In conclusion, the Sb-doped Li4Ti5O12 hollow spheres were successfully prepared through a two-step process. Benefiting from the unique structural features and Sb5+ doping, the as-prepared Sb-LTO hollow structures exhibit remarkable rate capability and cycling stability due to its higher electrical conductivity. Even at 10C, the Sb-LTO can deliver a specific capacity of 132.7 mA h g−1. As a result, the Sb-LTO electrodes derived from the proposed two-step method can be considered as a promising anode material for high-performance LIBs.Notes and references
- W. K. Pang, V. K. Peterson, N. Sharma, J. J. Shiu and S. h. Wu, Chem. Mater., 2014, 26, 2318–2326 CrossRef CAS.
- O. U. Rahman and S. Ahmad, RSC Adv., 2016, 6, 10584–10596 RSC.
- X. Lu, L. Gu, Y. S. Hu, H. C. Chiu, H. Li, G. P. Demopoulos and L. Q. Chen, J. Am. Chem. Soc., 2015, 137, 1581–1586 CrossRef CAS PubMed.
- H. Hashimoto, Y. Nishiyama, M. Ukita, R. Sakuma, M. Nakanishi, T. Fujii and J. Takada, Inorg. Chem., 2015, 54, 7593–7599 CrossRef CAS PubMed.
- W. Schmidt, P. Bottke, M. Sternad, P. Gollob, V. Hennige and M. Wilkening, Chem. Mater., 2015, 27, 1740–1750 CrossRef CAS.
- D. H. Long, M. G. Jeong, Y. S. Lee, W. Choi, J. K. Lee, I. H. Oh and H. G. Jung, ACS Appl. Mater. Interfaces, 2015, 7, 10250–10257 CAS.
- C. Wang, S. Wang, Y. B. He, L. k. Tang, C. p. Han, C. Yang, M. Wagemaker, B. H. Li, Q. H. Yang, J. K. Kim and F. Y. Kang, Chem. Mater., 2015, 27, 5647–5656 CrossRef CAS.
- Y. W. Shi, D. Y. Zhang, C. K. Chang, K. J. Huang and R. Holze, J. Alloys Compd., 2015, 639, 274–279 CrossRef CAS.
- X. Wang, D. Q. Liu, Q. H. Weng, J. W. Liu, Q. F. Liang and C. Zhang, NPG Asia Mater., 2015, 7, e171 CrossRef CAS.
- L. Wang, Y. M. Zhang, M. E. Scofield, S. Y. Yue, C. McBean, A. C. Marschilok, K. J. Takeuchi, E. S. Takeuchi and S. S. Wong, ChemSusChem, 2015, 8, 3304–3313 CrossRef CAS PubMed.
- M. Chen, W. Li, X. Shen and G. W. Diao, ACS Appl. Mater. Interfaces, 2014, 6, 4514–4523 CAS.
- C. Wang, S. Wang, L. K. Tang, Y. B. He, L. Gan, J. Li, H. D. Du, B. H. Li, Z. Q. Lin and F. Y. Kang, Nano Energy, 2016, 21, 133–144 CrossRef CAS.
- W. Li, M. Z. Chen, J. J. Jiang, R. Wu, F. Wang, W. J. Liu, G. C. Peng and M. Z. Qu, J. Alloys Compd., 2015, 637, 476–482 CrossRef CAS.
- E. M. Masoud and S. Indris, RSC Adv., 2015, 5, 108058–108066 RSC.
- W. Schmidt, P. Bottke, M. Sternad, P. Gollob, V. Hennige and M. Wilkening, Chem. Mater., 2015, 27, 1740–1750 CrossRef CAS.
- E. Pohjalainen, T. Rauhala, M. Valkeapää, J. Kallioinen and T. Kallio, J. Phys. Chem. C, 2015, 119, 2277–2283 CAS.
- T. F. Yi, Z. K. Fang, Y. Xie, Y. R. Zhu and S. Y. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 20205–20213 CAS.
- L. Peng, H. J. Zhang, L. Fang, Y. Zhang and Y. Wang, Nanoscale, 2016, 8, 2030–2040 RSC.
- Y. Ma, B. Ding, G. Ji and J. Y. Lee, ACS Nano, 2013, 7, 10870–10878 CrossRef CAS PubMed.
- T. F. Yi, S. Y. Yang, X. Y. Li, J. H. Yao, Y. R. Zhu and R. S. Zhu, J. Power Sources, 2014, 246, 505–511 CrossRef CAS.
- T. F. Yi, H. P. Liu, Y. R. Zhu, L. J. Jiang, Y. Xie and R. S. Zhu, J. Power Sources, 2012, 215, 258–265 CrossRef CAS.
- C. X. Qiu, Z. Z. Yuan, L. Liu, N. Ye and J. C. Liu, J. Solid State Electrochem., 2013, 17, 841–847 CrossRef CAS.
- X. Li, S. H. Tang, M. Z. Qu, P. X. Huang, W. Li and Z. L. Yu, J. Alloys Compd., 2014, 588, 17–24 CrossRef CAS.
- C. C. Yang, H. C. Hu, S. J. Lin and W. C. Chien, J. Power Sources, 2014, 258, 424–433 CrossRef CAS.
- T. F. Yi, Y. Xie, J. Shu, Z. H. Wang, C. B. Yue, R. S. Zhu and H. B. Qiao, J. Electrochem. Soc., 2011, 158, A266–A274 CrossRef CAS.
- T. F. Yi, Y. Xie, L. J. Jiang, J. Shu, C. B. Yue, A. N. Zhou and M. F. Ye, RSC Adv., 2012, 2, 3541–3547 RSC.
- N. S. Xu, X. Z. Sun, X. Zhang, K. Wang and Y. W. Ma, RSC Adv., 2015, 5, 94361–94368 RSC.
- L. Zhao, Y. S. Hu, H. Li, Z. X. Wang and L. Q. Chen, Adv. Mater., 2011, 23, 1385–1388 CrossRef CAS PubMed.
- W. Yang, X. Bai, T. Li, Y. Y. Ma, Y. X. Qi, L. W. Yin, H. Li, N. Lun and Y. J. Bai, RSC Adv., 2015, 5, 93155–93161 RSC.
- D. Li, Q. Qin, X. C. Duan, J. Q. Yang, W. Guo and W. J. Zheng, ACS Appl. Mater. Interfaces, 2013, 5, 9095–9100 CAS.
- W. Wang, B. Jiang, W. Y. Xiong, Z. Wang and S. Q. Jiao, Electrochim. Acta, 2013, 114, 198–204 CrossRef CAS.
- Y. J. Bai, C. Gong, Y. X. Qi, N. Lun and J. Feng, J. Mater. Chem., 2012, 22, 19054–19060 RSC.
| This journal is © The Royal Society of Chemistry 2016 |