Dan Zhu, Denghu Chang, Shaoyan Gan and Lei Shi
RSC Adv., 2016,6, 27983-27987
DOI:
10.1039/C6RA01799A,
Communication
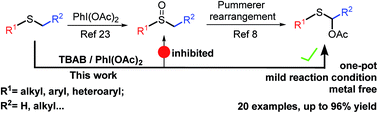
A novel metal-free approach to directly synthesize α-acyloxy sulfides from readily available alkyl sulfides utilizing the hypervalent (diacyloxyiodo)benzene and TBAB reagent combination is reported. This transformation is characterized by its broad substrate scope in moderate to excellent yields, convenient operating conditions and outstanding functional group tolerance.