Zinc-mediated diastereoselective assembly of a trinuclear circular helicate†
Abstract
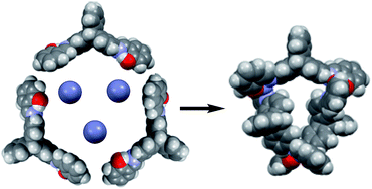
Coordination of a triptycene-based ditopic Schiff base ligand (H2L) with Zn2+ leads to the formation of a novel trinuclear circular species. A combination of 1H NMR spectroscopy, infrared spectroscopy, mass spectrometry and elemental analysis allow the unambiguous characterization of H2L and Zn3L3·8H2O. DFT molecular modelling has been used to study the possible isomers of the cyclic trinuclear Zn(II) complex that results from the combination of conformational and optical isomers of the ligand. A NOESY experiment demonstrated that in the trinuclear circular zinc(II) helicate the three isomers of the ligand have an anti configuration of N,O-donor domains and an s-cis configuration of their imine bonds. Besides, a UV-VIS absorption and fluorescence emission study of H2L and Zn3L3·8H2O has been performed.


 Please wait while we load your content...
Please wait while we load your content...