Multifunctional zinc oxide thin films for high-performance UV photodetectors and nitrogen dioxide gas sensors†
Abstract
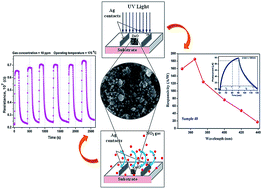
In order to find new approaches for sensor devices; ZnO based gas sensors and UV photodetectors with higher sensitivity and responsivity were fabricated. ZnO thin films were synthesized by using inexpensive successive ionic layers by an adsorption and reaction method (SILAR) on the amorphous glass substrate. The fabricated metal–semiconductor–metal (MSM) UV photoconductive detector shows excellent photoresponse with fast response and recovery times (18 s and 24 s) under UV illumination (wavelength −365 nm and power density −1.8 μW cm−2) at 5 V bias voltage. The detector shows an ohmic nature between metal semiconductor contacts with spectral responsivity 185 A W−1. Gas sensing performance for detecting NO2 gas was studied at a relatively low operating temperature of 175 °C and 20.52 response was observed for the optimized film for a 60 ppm gas concentration. The sensor has good repeatability along with a quick response time, whereas it has a relatively high recovery time. The sensor is highly selective towards NO2 gas as compared to other gases, and has a lower detection limit of 10 ppm at an operating temperature of 175 °C. The present study opens up possibilities for the extensive study of ZnO thin film based sensor devices using a simple chemical deposition method (SILAR).


 Please wait while we load your content...
Please wait while we load your content...