Synthesis of acrylic acid-allylpolyethoxy amino carboxylate copolymer and its application for removing calcium from crude oil
Shuaishuai Ma†
,
Zhilan Cai†,
Yuming Zhou*,
Shiwei Li and
Shuang Liang
School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, P. R. China. E-mail: ymzhou@seu.edu.cn; Fax: +86 25 52090617; Tel: +86 25 52090617
First published on 29th February 2016
Abstract
A novel environmentally friendly type of decalcifying agent acid, allylpolyethoxy amino carboxylate (AA–APEA), was synthesized and characterized by Fourier transform infrared spectrometry (FT-IR) and 1H-NMR. AA–APEA was used to remove calcium from Luning pipeline crude oil. The effects of several factors, such as molar ratio, dosage and reaction temperature on calcium removal efficiencies from crude oil were evaluated. The optimum conditions for calcium removal from crude oil were as follows: (a) molar ratio of AA–APEA was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) dosage of AA–APEA was 150 ppm, and (c) reaction temperature was 120 °C. Under these conditions, the removal efficiency of calcium from Luning pipeline crude oils was approximately 98.78%.
1, (b) dosage of AA–APEA was 150 ppm, and (c) reaction temperature was 120 °C. Under these conditions, the removal efficiency of calcium from Luning pipeline crude oils was approximately 98.78%.
Introduction
The global increase in unconventional crude-oil resources in recent years has resulted in increasing quantities of sour crudes, acidic crudes, or extra heavy crudes being produced.1 These crude oils have relatively high contents of metal contaminant. A number of important crude feedstocks, or the residual or deasphalted oil derived from them, contain levels of calcium that render them difficult to process using conventional refining techniques.2 The calcium which causes particular problems is present in these feedstocks as organically-bond compounds such as calcium naphthenate, which are not easily dissociated or removed by conventional water washing or desalting processes.3 These calcium compounds quickly decompose during typical catalytic operations, such as during hydroprocessing or during fluid catalytic cracking, causing rapid fouling or deactivation of the catalysts in the catalytic operation.4 Thus it is desirable to remove these compounds before additional processing. To date, various agents including carboxylic acids, phosphoric acid and its salts, combination of mineral acid, alkyl phosphate ester and an oxidant, hybrid of alkyl phosphate esters, alkyl carboxylic acid and mineral acids, and relatively high concentrations of sulfuric acid, are generally taught for removing organically-bound calcium and other metal contaminants from crude oil.5–11 However, these materials have low efficiency and environmental pollution problems. Especially, phosphonates can create serious problems such as insolubility of phosphonates complexes, and they are also susceptible to breakdown to form orthophosphates under the influence of hydrolysis and/or chlorination thereby increasing the potential of formation of calcium-phosphate deposits in the presence of excessive amounts of calcium.12–14 In addition, phosphonates, when reverted to orthophosphates, are potential nutrients for algae.15 Thus, there is a need for developing a new nonphosphorus and effective agent for the removal of metal contaminants, particularly calcium, from crude oil.Copolymer was developed in the late 1970s because of its strong complexation of multifunctional groups and superior dispersion characteristic of macromolecule.16 Several polymers have been used as a specific chemical additive agent in the crude oil desalting process such as poly (acrylic acid) derivative, poly-glycolic esters, poly thioglycolic acid and poly hydroxyl-carboxylic acids. This kind of chemical additive agent is high efficient and low-corrosive which attribute to their controllable pH range and much functional groups. In addition, some of them can perform as a metal corrosion inhibitor.10,11 However, when these polymer derivatives used as additive agents, the heavy precipitation problem arising in the reaction would lead to fouling of the processing equipments. Therefore, the current trend is develop an environmentally friendly “green” copolymer compose with several efficient and water-soluble functional groups such as amino (–NH2) and carboxylic (–COO−).
In present work, acrylic acid (AA)–allylpolyethoxy amino carboxylate (APEA), a novel non-phosphorous copolymer derived from allyloxy polyethoxy ether (APEG), was synthesized and used to remove calcium from Luning pipeline crude oil. Several effect factors such as molar ratio, dosage and reaction temperature were evaluated. Results showed that AA–APEA copolymer was successfully employed as a decalcifying agent for removing calcium from crude oil.
Experimental section
Materials
Allyloxy polyethoxy ether (APEG), L-asparagine, acrylic acid (AA) and ammonium persulfate were analytically pure grades and purchased from Aladdin Chemical Regent Co., Ltd. (Shanghai, China). The polyacrylic acid (PAA, 1800 MW), hydrolyzed polymaleic acid (HPMA, 600 MW) and polyepoxysuccinic acid (PESA) were technical grade and supplied by Jiangsu Jianghai Chemical Co., Ltd., (Changzhou, Jiangsu, P. R. China). Distilled water was used for all the studies.Preparation of APEA
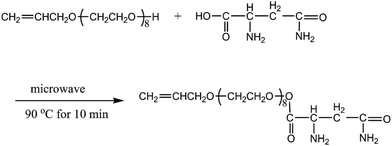
The carboxylic acid functionalization of the surface hydroxyl groups was realized with L-asparagine as shown in Fig. 1. Typically, 0.1 mol of L-asparagine was dissolved into 30 mL of HCl (3 wt%) solution, and then 0.1 mol of APEG was added into L-asparagine solution under continuous stirring. Subsequently, the reactant mixture was loaded into a Teflon autoclave, sealed, and placed in a microwave oven (MCR-3, 900 W). The autoclave was heated to 90 °C in 2 min and kept at this temperature for 10 min. After cooling to room temperature, the sample of APEA was obtained.Preparation of AA–APEA
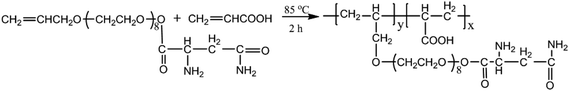
A five-neck round-bottom flask, equipped with a thermometer and a magnetic stirrer, was charged with 90 mL distilled water and 0.1 mol of APEA and heated to 70 °C with stirring under nitrogen atmosphere. Subsequently, 0.2 mol of AA in 20 mL distilled water (the molar ratio of APEA and AA was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and the initiator solution (3.5 g of ammonium persulfate in 20 mL distilled water) were added separately at constant flow rates over a period of 1.0 h. The reaction was then heated to 85 °C and maintained at this temperature for another 2 h, ultimately affording an aqueous polymer solution containing approximately 25.3% solid. At last, the product of AA–APEA was obtained. The preparation procedure of AA–APEA is shown in Fig. 2.
2) and the initiator solution (3.5 g of ammonium persulfate in 20 mL distilled water) were added separately at constant flow rates over a period of 1.0 h. The reaction was then heated to 85 °C and maintained at this temperature for another 2 h, ultimately affording an aqueous polymer solution containing approximately 25.3% solid. At last, the product of AA–APEA was obtained. The preparation procedure of AA–APEA is shown in Fig. 2.
Sample characterization
The samples were analyzed using a FT-IR spectroscopy (VECTOR-22, Bruker Co., Germany) in the region of 4000–500 cm−1. Prior to the measurement, the samples were dried under vacuum until reaching to a constant weight. The dried samples were pressed into the powder, mixed with KBr powder, and then compressed to make a pellet for FT-IR characterization. Structures of APEG, APEA, and AA–APEA were also explored by a Bruker NMR analyzer (AVANCE AV-500, Bruker, Switzerland) operating at 500 MHz. The calcium content of the oil samples were analyzed by means of an inductively coupled plasma mass spectrometry (ICP-MS, Thermo Elemental X7 Series).Procedure for removing calcium from crude oil
Removal experiments were carried out in a DPY-2 electric desalter instrument. 40 g of the Luning pipeline oil sample (the calcium content is 23.1 ppm) was placed in a constant temperature bath. After mixing uniformly, 2 mL of wash water and the desired dose of demulsifier were added to the mixture and stirred for 2 min at high stirring rate (about 2000 rpm) and then the mix crude oil was transferred into sample bottle which equipped with electrode. Subsequently, the desired dose of AA–APEA copolymer was added and the sample bottle was fixed in the oscillator and shaken for 5 min. The mixture was placed in a constant temperature bath at 120 °C for 10 min, and then placed in an electric field for 20 min. Finally, the mixture remained in the constant temperature for 15 min and the amounts of calcium in the oil were analyzed. Comparative experiments of removing calcium by using AA, APEA, PAA, HPMA and PESA samples were also carried out.Measuring the calcium content in crude oil
10.00 g of crude oil was weighed into a quartz crucible, slowly heated and then ignited. After combustion, the crude oil was calcined in a muffle furnace at 525 ± 25 °C to remove carbon residues. After the ash was allowed to cool, a small volume of distilled water was added to wet the ash and nitric acid and the hydrochloric acid solutions were then added. The crucible was heated slowly to dissolve the ash and the solution was removed quantitatively into a volumetric flask. The residual calcium content of crude oil was analyzed with an inductively coupled plasma mass spectrometry (ICP-MS).Results and discussion
Structure analysis of the copolymer
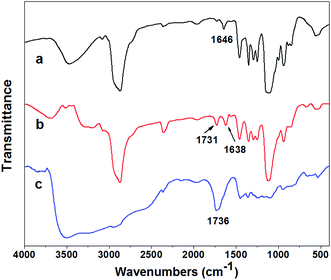
The FT-IR spectra of APEG (a), APEA (b), and AA–APEA (c) were shown in Fig. 3. The bands at 1646 cm−1 are from the stretching of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– groups. The 1731 cm−1 strong intensity absorption peak (–C
C– groups. The 1731 cm−1 strong intensity absorption peak (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in curve b reveals clearly that APEA has been synthesized successfully.17,18 The fact that the (–C
O) in curve b reveals clearly that APEA has been synthesized successfully.17,18 The fact that the (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) stretching vibration at 1646 cm−1 appears in curve a but disappears completely in curve c reveals that free radical copolymerization between APEA and AA has happened.
C–) stretching vibration at 1646 cm−1 appears in curve a but disappears completely in curve c reveals that free radical copolymerization between APEA and AA has happened.
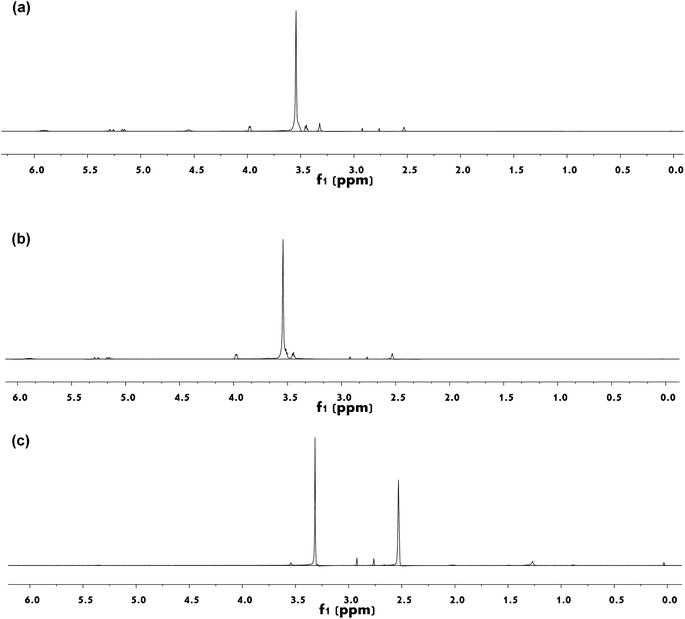
1H-NMR spectra for (a) APEG, (b) APEA, and (c) AA–APEA are shown in Fig. 4. APEG (Fig. 4(a)) ((CD3)2SO, δ, ppm): 2.50 (solvent residual peak of (CD3)2SO), 3.00–3.80 (–OCH2CH2–, ether groups), 3.80–6.00 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–, propenyl protons), and 4.40–4.60 (–OH, active hydrogen in APEG). APEA (Fig. 4(b)) ((CD3)2SO, δ, ppm): 2.50 (solvent residual peak of (CD3)2SO), 3.50–3.90 (–OCH2CH2–, ether groups), the disappeared peak at 4.41–4.60 ppm in Fig. 4(b) reveals that active hydroxyl group of APEG has reacted with L-asparagine,19 which confirms the FT-IR analysis of emerging 1731 cm−1 strong intensity absorption peak (–C
CH–CH2–, propenyl protons), and 4.40–4.60 (–OH, active hydrogen in APEG). APEA (Fig. 4(b)) ((CD3)2SO, δ, ppm): 2.50 (solvent residual peak of (CD3)2SO), 3.50–3.90 (–OCH2CH2–, ether groups), the disappeared peak at 4.41–4.60 ppm in Fig. 4(b) reveals that active hydroxyl group of APEG has reacted with L-asparagine,19 which confirms the FT-IR analysis of emerging 1731 cm−1 strong intensity absorption peak (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in Fig. 2(b). AA–APEA (Fig. 4(c)) (CD3)2SO, δ, ppm): 2.50 (solvent residual peak of (CD3)2SO, δ, 4.10–6.00 ppm in (b) double bond absorption peaks completely disappeared in (c). This reveals that free radical polymerization among APEA and AA has happened. From FT-IR and 1H-NMR analysis, it can conclude that synthesized AA–APEA has anticipated structure.
O) in Fig. 2(b). AA–APEA (Fig. 4(c)) (CD3)2SO, δ, ppm): 2.50 (solvent residual peak of (CD3)2SO, δ, 4.10–6.00 ppm in (b) double bond absorption peaks completely disappeared in (c). This reveals that free radical polymerization among APEA and AA has happened. From FT-IR and 1H-NMR analysis, it can conclude that synthesized AA–APEA has anticipated structure.
Influence of molar ratio of AA–APEA on calcium removal efficiency
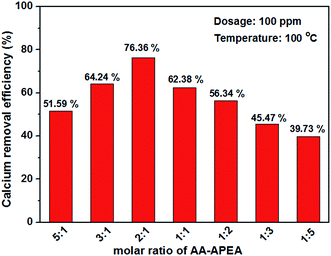
The data of calcium removal efficiency with different molar ratio of AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APEA at a dosage of 100 ppm and temperature of 100 °C are shown in Fig. 5. The difference of monomer molar ratio can result in a different conversion rate, and different proportion of the functional groups and polymer sequence structure. Polymers with different proportion of functional groups and sequence structure have different gravitation and repulsion to particles, and then resulting different ability to disperse particles. It can be seen from the data shown in Fig. 5 that molar ratio of AA
APEA at a dosage of 100 ppm and temperature of 100 °C are shown in Fig. 5. The difference of monomer molar ratio can result in a different conversion rate, and different proportion of the functional groups and polymer sequence structure. Polymers with different proportion of functional groups and sequence structure have different gravitation and repulsion to particles, and then resulting different ability to disperse particles. It can be seen from the data shown in Fig. 5 that molar ratio of AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APEA has an impact on the properties of AA–APEA and copolymer AA–APEA exhibits superior calcium removal efficiency when molar ratio of AA
APEA has an impact on the properties of AA–APEA and copolymer AA–APEA exhibits superior calcium removal efficiency when molar ratio of AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APEA is 2
APEA is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; thus we would rather select 2
1; thus we would rather select 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for further research.
1 for further research.
Influence of dosage of AA–APEA on calcium removal efficiency
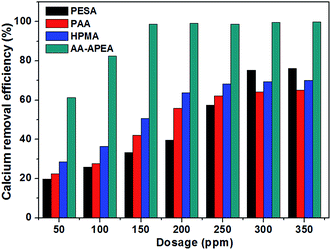
Fig. 6 shows the data of calcium removal efficiency under 100 °C in the presence of varying dosages of AA, APEA, AA/APEA hybrid (molar ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and AA–APEA. As can be seen, the calcium removal efficiency is only 12.32% without any chemical additive. However, the decalcification rate is increasing quite significantly when the copolymer of AA–APEA is added. The calcium removal efficiency can reach up to 95.69% when the dosage of AA–APEA in levels of up to 150 ppm, while the AA, APEA monomer and AA/APEA hybrid can only approach 20.11%, 25.53% and 40.24% for the same condition, respectively. The phenomenon that AA–APEA displayed the best calcium removal efficiency can be attributed to that AA–APEA copolymers contain more carboxylic (–COO−) and amino (–NH2) groups and have synergy between them.20,21 In addition, the decalcification rate does not obviously increase correspondingly when the concentration of AA–APEA exceeds 150 ppm. That is to say, the copolymer of AA–APEA is extremely effective for removing calcium from crude oil and results in much lower calcium content of the desalted oil.
1)) and AA–APEA. As can be seen, the calcium removal efficiency is only 12.32% without any chemical additive. However, the decalcification rate is increasing quite significantly when the copolymer of AA–APEA is added. The calcium removal efficiency can reach up to 95.69% when the dosage of AA–APEA in levels of up to 150 ppm, while the AA, APEA monomer and AA/APEA hybrid can only approach 20.11%, 25.53% and 40.24% for the same condition, respectively. The phenomenon that AA–APEA displayed the best calcium removal efficiency can be attributed to that AA–APEA copolymers contain more carboxylic (–COO−) and amino (–NH2) groups and have synergy between them.20,21 In addition, the decalcification rate does not obviously increase correspondingly when the concentration of AA–APEA exceeds 150 ppm. That is to say, the copolymer of AA–APEA is extremely effective for removing calcium from crude oil and results in much lower calcium content of the desalted oil.
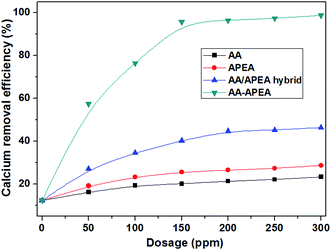 | ||
Fig. 6 Calcium removal efficiency in the presence of varying dosages of AA, APEA, AA/APEA hybrid (molar ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and AA–APEA. 1)) and AA–APEA. | ||
Effect of reaction temperature on calcium removal efficiency
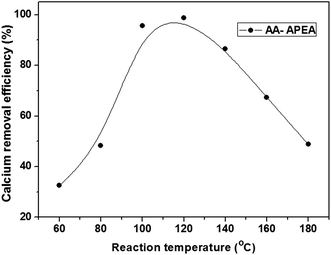
The experiments were to study the patterns of how reaction temperature affected the calcium removal efficiency when taking AA–APEA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a decalcifying agent, and the dosage of the AA–APEA sample was 150 ppm and the results are shown in Fig. 7. As can be seen, when the reaction temperature is 60 °C, the removal efficiency of calcium crude oil is 32.56%, and when the temperature is increased up to 120 °C, the removal efficiency of calcium increase to 98.78%. The result may attributed to that the increasing of temperature could make the copolymer gain much energy and stretching, make the effective groups more active and enhance its decalcifying property. However, when the temperature raises from 120 °C to 180 °C, the corresponding efficiency decreases from 98.78% to 48.77%, about 40% loss in decalcifying process. This can be ascribed that the high temperature is not benefit for the coalescence of water droplets and the dissolved calcium compounds are difficult to separate with discharge water promptly. In addition, the R–COOH might react with H2N–R groups to form lactams and the bi-functional groups are being converted into a non-chelating lactam moiety at temperatures greater than 120 °C. Therefore, the optimum temperature was 120 °C and the calcium removal efficiency from crude oil is 98.78%.
1) as a decalcifying agent, and the dosage of the AA–APEA sample was 150 ppm and the results are shown in Fig. 7. As can be seen, when the reaction temperature is 60 °C, the removal efficiency of calcium crude oil is 32.56%, and when the temperature is increased up to 120 °C, the removal efficiency of calcium increase to 98.78%. The result may attributed to that the increasing of temperature could make the copolymer gain much energy and stretching, make the effective groups more active and enhance its decalcifying property. However, when the temperature raises from 120 °C to 180 °C, the corresponding efficiency decreases from 98.78% to 48.77%, about 40% loss in decalcifying process. This can be ascribed that the high temperature is not benefit for the coalescence of water droplets and the dissolved calcium compounds are difficult to separate with discharge water promptly. In addition, the R–COOH might react with H2N–R groups to form lactams and the bi-functional groups are being converted into a non-chelating lactam moiety at temperatures greater than 120 °C. Therefore, the optimum temperature was 120 °C and the calcium removal efficiency from crude oil is 98.78%.
Comparison with the other polymers
Fig. 8 illustrates the decalcifying ability of AA–APEA compared with that of currently common phosphorous-free polymers at a reaction temperature of 120 °C. The data shown in Fig. 8 indicate that the ability to remove calcium from crude oil followed the order AA–APEA > HPMA > PAA > PESA (under 250 ppm). As can be seen, the calcium removal efficiency for copolymer AA–APEA can reach 98.78% at the dosage of 150 ppm, while HPMA, PAA and PESA can only approach 50.62%, 42.15% and 33.23%, respectively. In addition, the calcium removal efficiency does not obviously increase with the increasing concentrations after the concentration exceeds 150 ppm. However, HPMA, PAA and PESA polymers also have relative superior ability on the removal of calcium from crude oil, with 68.13%, 62.17% and 57.33% at a level of 250 ppm, respectively. It is also worth mentioning that the PESA displays better calcium removal efficiency than both of HPMA and PAA when the dosage overcoming 300 ppm. The results may ascribed that the amino (–NH2) groups exist in AA–APEA copolymers generating more active-sites and have some synergy affects on the decalcifying performance. Therefore, it can be concluded from above that the copolymer AA–APEA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displays the best ability to remove calcium from crude oil among those polymers investigated, namely HPMA, PAA and PESA.
1) displays the best ability to remove calcium from crude oil among those polymers investigated, namely HPMA, PAA and PESA.
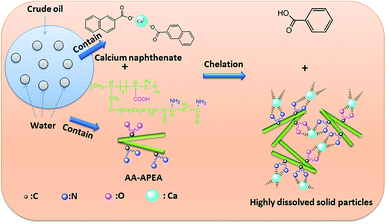
Proposed mechanism for decalcification
It was previously discovered that the addition of water-soluble AA–APEA copolymer to crude oil can significantly reduce the amount of calcium when it is run through an electric desalter instrument. Based on the above results, a possible mechanism of removing calcium from crude oil was proposed and presented in Fig. 9. As can be seen, AA–APEA is a structurally well-defined biblock copolymer with a large number of carboxyl and amino groups. It can be considered as a member of a broad class of multidentate chelating ligands which complex or coordinates metal ions and these metal complexes are very stable.22–24 Herein, crude oil was mixed with an aqueous solution of AA–APEA and produces an aqueous/organic multi-phase mixture. With the vigorous mixing of the oil and water, the acids formed after hydrolysis of the chemical compound and the calcium ions of calcium naphthenate were substituted for H+ by acid displacement reaction. Once confronted with calcium ions, the carboxyl and amino groups of AA–APEA can recognize and react with the positively charged ions, which would leads to the spontaneous formation of AA–APEA–Ca complexes. AA and APEA blocks are hydrophilic chain segments, thus the AA–APEA–Ca complexes are also water soluble and the calcium in the organic phase is transported across the interface between the two phases and dissolves in the aqueous phase, and therefore it could be washed and removed from the crude oil by an electric desalting process. In this case, the calcium removal efficiency is more pronounced with more carboxyl and amino containing groups.Conclusions
In summary, water-soluble monomer APEA was produced from APEG and L-asparagine by esterification via the microwave technique rout. Phosphorous free copolymer of AA–APEA was synthesized with different molar ratios in this study and the synthesized copolymers were characterized by FT-IR and further conformed by 1H-NMR. The majority of inorganic metals in crude oil can be removed with an electric desalting process. However, the organically-bond compounds such as calcium naphthenate are oil-soluble and thus they cannot be removed by the electric desalting process. After copolymer of AA–APEA was introduced to crude oil, removal efficiencies of calcium from Luning pipeline crude oil can reach approximately 98.78% at the optimum conditions as follows: (a) molar ratio of AA–APEA was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) dosage of AA–APEA was 150 ppm, and (c) reaction temperature was 120 °C. Compared to the recent nonphosphorus polymer of PAA, HPMA and PESA, AA–APEA possessing carboxyl and amino groups shows superior calcium removal efficiency from crude oil. The decalcification mechanism is proposed that displacement and chelation happened between AA–APEA and calcium ion, and the water soluble AA–APEA–Ca complexes are formed; hence the calcium is removed via the water phase. Therefore, AA–APEA copolymer is believed to represent a potentially environmental decalcifying agent of crude oil.
1, (b) dosage of AA–APEA was 150 ppm, and (c) reaction temperature was 120 °C. Compared to the recent nonphosphorus polymer of PAA, HPMA and PESA, AA–APEA possessing carboxyl and amino groups shows superior calcium removal efficiency from crude oil. The decalcification mechanism is proposed that displacement and chelation happened between AA–APEA and calcium ion, and the water soluble AA–APEA–Ca complexes are formed; hence the calcium is removed via the water phase. Therefore, AA–APEA copolymer is believed to represent a potentially environmental decalcifying agent of crude oil.
Acknowledgements
The authors are grateful to the financial supports of National Natural Science Foundation of China (Grant No. 21376051, 21306023, 21106017), Natural Science Foundation of Jiangsu (Grant No. BK20131288), Fund Project for Transformation of Scientific and Technological Achievements of Jiangsu Province of China (Grant No. BA2014100) and the Fundamental Research Funds for the Central Universities (3207045301, T15192014, KYLX_0161).References
- D. Zhang, Pet. Refin. Eng., 2002, 32, 1–6 CAS.
- D. L. Kuehne, L. P. Hawker and D. C. Kramer, US Pat., 6905593, 2005.
- L. N. Kremer, J. J. Weers and C. Sandu, US Pat., 8425765, 2013.
- M. Subramaniyam, US Pat., 8685233, 2014.
- R. B. Duke, US Pat., 4439345, 1984.
- A. Goliaszewski, D. Engel and R. May, US Pat., 6905593, 2005.
- F. J. Krambeck, C. T. Lam and P. H. Schipper, US Pat., 4645589, 1987.
- G. R. Norman, US Pat., 4432865, 1984.
- R. Powell, US Pat., 2778777, 1957.
- M. Subramaniyam, US Pat., 8440072, 2013.
- M. Subramaniyam, US Pat., 8840781, 2014.
- B. R. Zhang, L. Zhang, F. T. Li, W. Hu and P. M. Hannam, Corros. Sci., 2010, 52, 3883–3890 CrossRef CAS.
- A. Tsortos and G. H. Nancollas, J. Colloid Interface Sci., 2002, 250, 159–167 CrossRef CAS PubMed.
- Z. Amjad, Langmuir, 1989, 5, 1222–1225 CrossRef CAS.
- A. A. Koelmans, A. Van der Heijde, L. M. Knijff and R. H. Aalderink, Water Res., 2001, 35, 3517–3536 CrossRef CAS PubMed.
- M. M. Reddy and G. H. Nancolla, Desalination, 1973, 12, 61–73 CrossRef CAS.
- C. G. Fu, Y. M. Zhou, G. Q. Liu, J. Y. Huang, W. Sun and W. D. Wu, Ind. Eng. Chem. Res., 2011, 50, 10393–10399 CrossRef CAS.
- K. Cao, Y. M. Zhou, G. Q. Liu, H. C. Wang and W. Sun, J. Appl. Polym. Sci., 2014, 131, 40193 Search PubMed.
- H. C. Wang, G. Q. Liu, J. Y. Huang, Y. M. Zhou, Q. Z. Yao, S. S. Ma, K. Cao, Y. H. Liu, W. D. Wu, W. Sun and Z. J. Hu, Desalin. Water Treat., 2015, 53, 8–14 CrossRef CAS.
- S. C. Wang, J. Y. Yang and X. R. Xu, Fuel, 2011, 90, 987–991 CrossRef CAS.
- S. C. Wang, X. R. Xu, J. Y. Yang and J. S. Gao, Fuel Process. Technol., 2011, 92, 486–492 CrossRef CAS.
- A. E. Martell, J. Am. Chem. Soc., 1965, 87, 2527–2528 CrossRef.
- G. Leaf, Nature, 1965, 207, 564–565 CrossRef.
- F. Dwyer, Chelating agents and metal chelates, Elsevier, 2012 Search PubMed.
Footnote |
| † S. S. Ma and Z. L. Cai contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |