A self-directed and reconstructible immobilization strategy: DNA directed immobilization of alkaline phosphatase for enzyme inhibition assays†
Ye Yang,
Ping Su,
Kangle Zheng,
Ting Wang,
Jiayi Song and
Yi Yang*
Beijing Key Laboratory of Environmentally Harmful Chemical Analysis, College of Science, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: yangyi@mail.buct.edu.cn
First published on 7th April 2016
Abstract
This study focused on the development of a common method for the reversible and self-directed immobilization of enzymes based on a DNA-directed immobilization (DDI) technique. The successful anchoring of alkaline phosphatases to the surfaces of magnetic nanoparticles was confirmed using confocal laser scanning microscopy, thermogravimetric analysis and a vibrating sample magnetometer. The length of the DNA linker was optimized, with a base number of 24 providing maximum efficiency for the enzymolysis. Notably, the surface of alkaline phosphatase-functionalized magnetic nanoparticles could be regenerated using a mild dehybridization process of DNA. Furthermore, the resulting single-stranded probe DNA-modified magnetic nanoparticles could be reused to immobilize the alkaline phosphatase, which suggested that the DNA-functionalized surface of magnetic nanoparticles exhibited good reversibility. The biological activity of anchored alkaline phosphatases is evaluated in an enzyme inhibition assay. The results revealed that theophylline exhibited greater inhibitory activity than L-phenylalanine. The proposed protocol demonstrates a simple, mild and economic pathway for fabricating enzyme modified nanomaterial and can be applied in the high-throughput screening of inhibitors.
1. Introduction
Enzymes are biocatalysts that have been used in a variety of research areas and industries because of their high activity, selectivity and specificity. However, the application of native enzymes has been limited for stability and operational reasons. The immobilization of enzymes has been introduced as an effective strategy to improve the storage and operational stability of enzymes,1 as well as increasing the ease with which they can be used in multi-enzyme cascade processes and recovered from complex reaction mixtures.2,3 Immobilized enzymes have shown great promise in a number of different areas, including biochemistry,4–6 biosensors,7 clinical diagnosis8,9 and the removal of pollutants.10,11 Several different immobilization protocols have been studied, such as adsorption, entrapment, covalent bonding, cross-linking and affinity immobilization.12 Given that there are several advantages and disadvantages associated with the current immobilization methods, plenty of scope remains for the development of new techniques for the immobilization of enzymes.DNA-directed immobilization (DDI) is a mild and versatile chemical technique for specific immobilization of biological molecules based on the specific Watson–Crick base pairing (A–T, G–C) of DNA molecules. It has already been applied for the immobilization of proteins,13,14 antibodies15,16 and cells.17,18 Compared with other immobilization methods, DDI has several merits. For example, DNA can be readily obtained by numerous chemical processes and exhibits good mechanical rigidity and high physicochemical stability.19–21 Furthermore, the immobilization of biomolecules through DDI allows them to maintain their original activity because they are fixed onto the surfaces of a suitable support via DNA strands. In contrast, the direct anchoring of a biomolecule to a solid support through covalent or non-covalent interactions can restrict its conformational freedom and denature (partial) its structure.22,23 The DDI technique provides a mild self-assembly process for the binding of biomolecules and shows great specificity and selectivity.24,25 Moreover, immobilized biomolecules prepared using the DDI method can be reconstituted because of the reversible nature of the DNA hybridization process.26 This particular feature could potentially be used to reduce the number of steps and overall cost of each reaction.
In this study, we have developed a common method for the reversible immobilization of enzymes based on DDI technique. Alkaline phosphatase (ALP) was used as a model enzyme and anchored to the surface of magnetic nanoparticles (MNPs) using a DDI method. ALPs are dimeric phosphomonoesterases that exist in a wide range of organisms, and these enzymes can catalyze the hydrolysis of the phosphomonoesters present on a wide range of substrates.27,28 ALPs play significant roles in analytical chemistry and sensors because of their ease of operation, low cost and high activity.29,30 MNPs were selected as a suitable support in this study for the immobilization of an ALP enzyme because of their large surface area, favorable dispersity, good biocompatibility and special magnetism, which would allow them to be rapidly removed from the reaction mixture.31 Various characterization techniques were used in this study to confirm the formation of the immobilized ALP MNPs. High performance liquid chromatography (HPLC) was applied to evaluate the enzymolysis performance of the immobilized enzyme. The inhibition kinetics of the immobilized ALP MNPs were evaluated using theophylline and L-phenylalanine.
2. Experimental
2.1. Materials
Alkaline phosphatase (ALP) from bovine intestinal mucosa (EC 3.1.3.1, 10 U mg−1) and 4-nitrophenylphosphate were purchased from Sigma-Aldrich (St Louis, MO, USA). 3-Aminopropyltriethoxysilane (APTES), L-phenylalanine (L-Phe), tetraethyl orthosilicate (TEOS), glutaraldehyde (50 wt%) and methanol (HPLC grade) were purchased from J&K Scientific (Beijing, China). Theophylline and 4-nitrophenol were obtained from Acros Organics (Geel, Belgium) and AccuStandard (New Haven, CT, USA), respectively. All of the DNA molecules used in this study were synthesized and purified by Shanghai Sangon Biological Science & Technology Co., Ltd (Shanghai, China). The details of the DNA sequences used in this study are listed in Table S1.† Tris (2-carboxyethyl) phosphine (TCEP), sulfosuccinimidyl-4-(N-maleimido-methyl) cyclohexane-1-carboxylate (sulfo-SMCC) and BSA were purchased from Aladdin (Beijing, China). GeneFinder was obtained from Zeesan Biotech Co., Ltd (Xiamen, China). Magnesium chloride hexahydrate was purchased from Xilong Huagong Co., Ltd (Shantou, China). All of the other reagents used in this study were purchased as the analytical grade from Beijing Chemical Reagent Co., Ltd. (Beijing, China). Buffer A (10 mM sodium phosphate buffer, pH 7.4, 0.1 M NaCl, 0.05% Tween-20) was used in this experiment.2.2. Conjugation of APTES@SiO2@Fe3O4 with probe DNA strands
The Fe3O4 magnetic nanoparticles (MNPs) used in the current study were fabricated using a solvothermal reduction method.32 Briefly, FeCl3·6H2O (1.35 g) and anhydrous sodium acetate (3.6 g) were dissolved in ethylene glycol (40 mL), and the resulting mixture was vigorously stirred to give a homogenous yellow solution. The solution was then transferred to a Teflon-lined stainless steel autoclave and heated at 200 °C for 8 h. The mixture was cooled to room temperature and filtered to give a black product, which was then washed several times with water and ethanol before being dried under vacuum at 50 °C for 3 h.SiO2@Fe3O4 core–shell nanoparticles were synthesized using a sol–gel method as previously ref. 33 Briefly, Fe3O4 MNPs (1 g), aqueous NH3 (5 mL) and water (50 mL) were mixed ethanol (150 mL) under ultrasonic in a 500 mL three-necked flask for 15 min. A solution of TEOS (2 mL) in ethanol (50 mL) was then added to the reaction in dropwise manner, and the resulting mixture was stirred for 8 h at room temperature. The SiO2@Fe3O4 nanoparticles were separated from the reaction mixture using an external magnet and washed sequentially with 1 M HCl and water, before being dried under vacuum at 50 °C for 3 h.
APTES@SiO2@Fe3O4 was synthesized according to the following procedure. Silica coated Fe3O4 nanoparticles were dispersed in a toluene solution containing 5% (v/v) APTES, and the resulting mixture was heated under microwave irradiation at 100 °C for 5 h under an atmosphere of nitrogen in a Discover SP microwave reactor (CEM, Matthews, NC, USA). The resulting aminated magnetic beads were collected with an external magnet and rinsed three times with ethanol before being dried under vacuum at 50 °C for 3 h.
Single-stranded probe DNA (sspDNA) was connected to the surface of APTES@SiO2@Fe3O4 using glutaraldehyde as a cross-linking reagent. After being washed three times with PBS (20 mM, pH 8.0), the APTES@SiO2@Fe3O4 nanoparticles (5 mg) were incubated with a glutaraldehyde buffer solution (5 wt%) under continuous stirring for 2.5 h, and then rinsed with PBS. One microliter of a buffer solution containing the 5′-aminated DNA probe (2 μM) was added to the glutaraldehyde-bound Fe3O4 nanoparticles, and the mixture was incubated at 37 °C for 3.5 h. The resulting sspDNA anchored MNPs (sspDNA-MNPs) were washed 5 times with 20 mM PBS (pH 8.0) to remove any unreacted sspDNA, before being treated with an ethanol amine solution (1 mM) for 30 min to neutralize the remaining aldehyde groups of the sspDNA-MNPs. Finally, the pDNA modified MNPs were treated with a 1% BSA solution (in buffer A) for 30 min at 37 °C. In this case, the BSA solution was used as a blocking reagent to reduce the nonspecific adsorption sites of the MNPs. The resulting sspDNA-MNPs were washed with buffer A and immersed in 10 mM tris–HCl buffer (pH 8.0, 0.1 M NaCl) and stored at 4 °C for further use.
2.3. Synthesis of ALP-cDNA conjugates
Alkaline phosphatase (ALP) was conjugated to the 5′-thiol modified single-stranded complementary DNA (sscDNA) using sulfo-succinimidyl-4-(N-maleimido-methyl)-cyclohexan-1-carboxylte (sulfo-SMCC) as a biofunctional coupling reagent according to a slightly modified version of a previously reported procedure.23,34 Briefly, a solution of thiol-sscDNA in PBS (150 μL) was mixed with a 30 mM aqueous solution of TCEP (60 μL), and the resulting solution was incubated at 25 °C for 1 h with gentle shaking to cleave the disulfide bonds. The mixture was then purified six times over Amicon-3K using buffer A without Tween-20. ALP (2 mg) and sulfo-SMCC (1 mg) were dissolved in buffer A without Tween-20 (400 μL) by vortex mixing and the resulting mixture was transferred to a roller at 25 °C for 1 h. The mixture was subsequently centrifuged to remove any insoluble, unreacted sulfo-SMCC. The supernatant was purified 6 times over Amicon-10K using buffer A without Tween-20 as an eluent. The purified material was then mixed with the thiol-sscDNA solution described above. The resulting mixture was reacted at 37 °C for 48 h. To remove the excess thiol-sscDNA, the conjugate was purified 6 times over Amicon-10K using buffer A without Tween-20 as an eluent.2.4. Preparation of immobilized ALP on MNPs using a DDI strategy
Alkaline phosphatase was immobilized on bio-functionalized MNPs using a DNA-directed immobilization (DDI) method. Briefly, sspDNA-MNPs (5 mg) were dispersed in a 1.6 mg mL−1 solution of the ALP-sscDNA conjugate (1.25 mL), and the resulting suspension was placed on a shaker for 3 h at 37 °C. The anchored ALP magnetic nanoparticles (ALP-DNA-MNPs) were washed thoroughly with buffer A to remove the unbonded ALP-sscDNA conjugate and then stored at 4 °C for further study.The ALP-DNA-MNPs were dispersed in a 0.05 M aqueous solution of NaOH at room temperature for 3 min to remove the DNA-enzyme conjugate and regenerate the bio-functionalized surface of the sspDNA-MNPs.
2.5. Characterization
An Agilent 1100 Series HPLC system (Agilent, Santa Clara, CA, USA) equipped with an ultraviolet detector operating at a wavelength of 311 nm and an Agilent Eclipse XDB-C18 column (150 × 4.6 mm, i.d., 5 μm particle size) was used to detect the effect of the enzymolysis of the immobilized ALP. A mixture of methanol and water (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, v/v) was used as the mobile phase at a flow rate of 1 mL min−1. Magnetic characterization was performed on a Lake Shore 7410 vibrating sample magnetometer (VSM, Westerville, OH, USA). A Mettler Toledo 1100SF thermogravimetric analyzer (Columbus, OH, USA) was used for the thermogravimetric analysis (TGA) of the samples. The TGA experiments were conducted at temperatures from 25–1000 °C at a heating rate of 10 °C min−1 under a nitrogen atmosphere. The immobilization of ALP and sspDNA on the functionalized Fe3O4 nanoparticles was confirmed by confocal laser scanning microscopy (CLSM) on a Leica TCS SP5II CLSM system (Wetzlar, Germany).
60, v/v) was used as the mobile phase at a flow rate of 1 mL min−1. Magnetic characterization was performed on a Lake Shore 7410 vibrating sample magnetometer (VSM, Westerville, OH, USA). A Mettler Toledo 1100SF thermogravimetric analyzer (Columbus, OH, USA) was used for the thermogravimetric analysis (TGA) of the samples. The TGA experiments were conducted at temperatures from 25–1000 °C at a heating rate of 10 °C min−1 under a nitrogen atmosphere. The immobilization of ALP and sspDNA on the functionalized Fe3O4 nanoparticles was confirmed by confocal laser scanning microscopy (CLSM) on a Leica TCS SP5II CLSM system (Wetzlar, Germany).
2.6. ALP-DNA-MNPs activity assay and inhibition study
The catalytic activity of the immobilized ALP towards the cleavage of the phosphomonoesters of 4-nitrophenylphosphate (4-NPP) was measured spectrophotometrically. 4-NPP and the resulting enzymolysis product 4-nitrophenol35 can be measured directly without derivation because of their strong ultraviolet absorbance characteristics. The substrate was dissolved in the enzymolysis buffer (20 mM carbonate buffer containing 5 mM MgCl2) and diluted to a variety of different concentrations. The ALP-DNA-MNPs (0.5 mg) were subsequently mixed with different concentrations of the substrate solution (1 mL) under gentle shaking. Different concentrations of the inhibitors were added to the substrate solution, and the resulting mixtures were subjected to constant stirring. The ALP anchored magnetic nanoparticles were subsequently isolated using an external magnetic field, and the remaining supernatant was analyzed by HPLC. Based on the peak area of 4-nitrophenol, it was possible to determine the optimal enzymolysis conditions. The percentage inhibition (PI) was evaluated using the following formula:| PI (%) = 1 − An/A0 | (1) |
3. Results and discussion
3.1. Characterization of DNA-directed immobilization of ALP on MNPs
The immobilization of ALP onto the MNPs was achieved using the DDI method and the enzymatic activity of the immobilized material was measured according to the process shown in Scheme 1. The Fe3O4 MNPs were initially synthesized using a one-step solvothermal method and then coated with a layer of nonporous silica though the hydrolysis and condensation of TEOS. The SiO2@Fe3O4 nanoparticles were modified with primary amine groups using APTES in anhydrous toluene, and the resulting aminated silica-coated MNPs were then functionalization with glutaraldehyde. To fabricate an anchor for the immobilization of ALP, 5′-aminated probe DNA (sspDNA) was grafted onto the surface of the functionalized magnetic beads via an interaction between the amine and the aldehyde moieties of the different components. The binding of sspDNA onto the functionalized magnetic beads was confirmed by confocal laser scanning microscopy (CLSM). To enhance the fluorescence efficiency of the DNA, the sspDNA was labeled with a FAM. Fig. 1a shows a fluorescence microscope image of the FAM-sspDNA-MNPs. The image in the figure shows fluorophore that the FAM-sspDNA-MNPs exhibited strong green fluorescence when they were subjected to excitation at 495 nm. Compared with the bright field image (Fig. 1b) of FAM-sspDNA-MNPs, the fluorescence microscope image of this material indicated that the signal was emitted from the FAM labeled sspDNA-MNPs rather than the FAM-sspDNA residue in the solution. A control experiment was also conducted where the aldehyde-functionalization step was omitted from the synthetic process. As shown in Fig. 1c, no fluorescence signal was observed in the control experiment. This result therefore suggested that there were no measurable interactions between the MNPs and the probe DNA in the absence of glutaraldehyde. Taken together, these results demonstrate that the sspDNA was immobilized on the functionalized MNPs through the cross-linker glutaraldehyde, rather than physical absorption. | ||
| Fig. 1 (a) CLSM image of FAM-sspDNA-MNPs, (b) bright field image of FAM-sspDNA-MNPs and (c) control experiment: adsorption of FAM-sspDNA onto MNPs without glutaraldehyde. | ||
We subsequently investigated the use of sulfo-SMCC as a heterobispecific cross-linker for synthesis of a DNA-enzyme conjugate. The maleimide and n-hydroxysuccinimide ester groups of sulfo-SMCC can react with the thiol and Lys residues of sscDNA and ALP, respectively. The sscDNA-ALP conjugates were subsequently attached to the surfaces of complementary probe DNA-modified MNPs using a specific Watson–Crick hybridization to allow for the immobilization of ALP. The resulting material was subjected to TGA (Fig. S1†), which showed that the loss of weight from the ALP-DNA-MNPs was more dramatic than that of APTES@SiO2@Fe3O4 nanoparticles, most likely because of the immobilization of DNA and ALP onto MNPs. The magnetic properties of these newly synthesized magnetic nanomaterials were investigated using a VSM at room temperature, and the resulting hysteresis curves are shown in Fig. S2.† None of the samples showed any coercivity or remanence, which confirmed that the magnetic beads were superparamagnetic. The magnetization saturation values of Fe3O4, SiO2@Fe3O4 and the ALP-DNA-MNPs were 88.15, 74.34 and 54.57 emu g−1, respectively. This dramatic decrease in the magnetic response was attributed to the shielding effects of the nonmagnetic coatings (i.e., the SiO2 shell, DNA and ALP) on the surfaces of the MNPs. Furthermore, all of the samples could be dispersed in solution with gentle shaking and completely separated within 20 s (inset of Fig. S2†). The modified MNPs also exhibited excellent magnetic responsivity and dispersibility properties, which could be advantageous for further applications.
GeneFinder, which is a fluorescent dye for the detection of nucleic acids, was used to confirm the hybridization of the DNA. GeneFinder is a sensitive dye that is capable of intercalating with nucleic acids. Notably, this dye exhibits weak fluorescence in solution but shows strong green fluorescence when it is combined with double-stranded DNA. Fig. 2a shows a CLSM image of the ALP-DNA-MNPs following the addition of the GeneFinder dye. This image clearly shows that there were a large amount of small fluorescent dots on the surfaces of the ALP-DNA-MNPs. A control experiment was also conducted where the aminated silica-coated Fe3O4 nanoparticles were directly reacted with sscDNA-ALP conjugates without being modified by sspDNA. In this case, very few fluorescent dots were observed (Fig. 2c), which suggested that the hybridization of the DNA occurred on the surface of the sspDNA-modified MNPs. The immobilization of ALP onto the MNPs was confirmed by the enzymatic hydrolysates of the ALP-DNA-MNPs (Fig. 2d) and the results of a control experiment (Fig. 2e). Typical chromatograms for these experiments revealed that the sscDNA-ALP conjugates could be successfully anchored to the sspDNA-MNPs and subsequently used to catalyze the hydrolysis of 4-NPP, whereas the amino-modified MNPs could not be used in this way. This result indicated that there was no discernible interaction between the sscDNA-ALP conjugates and the amino-modified MNPs in the absence of the anchored sspDNA. Taken together, these results confirmed that ALP was successfully immobilized on the MNPs using the DDI strategy and that it retained its specificity and activity. This protocol is therefore feasible for using ALP-DNA-MNPs to study the activity and inhibition of the immobilized enzyme.
3.2. Effect of the length of the DNA
Different strand lengths of DNA were fixed onto the surfaces of the functionalized MNPs, and the hybridized of these different DNAs with complementary DNA-enzyme conjugates was investigated. Four different strand lengths of DNA were studied in this experiment, including DNA lengths of 12 (p12), 24 (p24), 33 (p33) and 42 (p42) bases. The results of a series of enzymolysis experiments using these different strand lengths revealed that a p24 gave the maximal efficiency (Fig. 3).When the p12 DNA strand was used for the immobilization of ALP, the enzyme was much closer to the surface of the MNPs. This led to a restriction in the conformational freedom of ALP, which resulted in a decrease in its activity. In addition, the p12 DNA stand would rapidly form hairpins in solution, and it might form a self-protected structure which could inhibit hybridization. The p33 and p42 DNA stands showed lower enzymolysis efficiencies than the p24 strand, and this can be attributed to the formation of interstrand bridges. This strong interaction between the neighbouring DNA strands will limit accessibility of the bases and prevents efficient hybridization.36 Besides, p33 and p42 DNA stands are longer than p24 stand, and the mechanical rigidity of longer DNA stand is lower than short DNA stand. In this case, instead of extending upwards into solution and away from the MNPs, the longer DNA strands would show a greater tendency towards laying on the surface of the MNPs, which hindered the hybridization of the pDNA with the cDNA-ALP conjugates.
3.3. Optimization of enzymolysis conditions
Several key parameters for the enzymolysis process, including the temperature, pH, incubation time and substrate concentration were optimized to improve the procedure.The temperature is one of the most important factors for an enzymatic hydrolysis. The influence of the temperature on the hydrolysis of 4-NPP was evaluated for temperatures in the range of 20–60 °C. The results revealed that enzymolysis efficiency initially increased with increasing temperature up to 40 °C, and that it subsequently declined as the temperature was increased further to 60 °C (Fig. 4a). This result therefore demonstrated that a temperature of 40 °C was optimal for the enzymatic hydrolysis of 4-NPP. Furthermore, this result was similar to a previous result from the literature.37 The relative activities of ALP at different pH values are depicted in Fig. 4b. Both extremes of overly high or low pH were found to be unfavorable for the immobilized ALP, and the optimum pH for the enzymatic activity was determined to be 9.5. The influence of the incubation time on the yield of 4-nitrophenol was also investigated and the results are shown in Fig. 4c. The results of these experiments revealed that there was a significant increase in the yield of the hydrolysis as the incubation time increased from 30 to 90 min, with the yield reaching a plateau for incubation times over 90 min. Based on these results, an incubation time of 90 min was selected as the optimal incubation time for subsequent experiments. The concentration of the substrate was investigated over a range of 0.001 to 2 mg mL−1. The results showed that the enzymolysis efficiency increased as the concentration of 4-NPP increased up to 0.5 mL−1, and that further increases in the concentration led to much less pronounced increases in the relative activity (Fig. 4d). This result was attributed to the saturation of the binding capacity of the enzyme. Based on these results, a 4-NPP concentration of 0.5 mL−1 was selected as the optimal concentration for further evaluation.
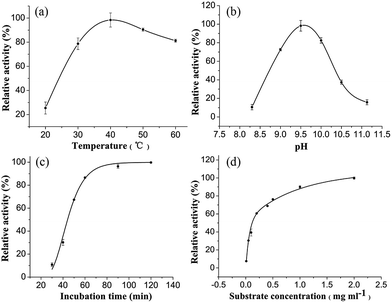 | ||
| Fig. 4 Effect of temperature (a), pH (b), incubation time (c) and substrate concentration (d) on the performance of the immobilized ALP. | ||
The repeatability of the ALP-DNA-MNPs was evaluated by carrying out the enzymatic hydrolysis with the same immobilized ALP. The results of these experiments revealed that the relative standard deviation (RSD) was 3.42% (n = 5).
3.4. Regeneration of immobilized ALP
A significant advantage of using the DDI method for immobilization of an enzyme is the capacity for surface regeneration, as illustrated in Fig. 5a. Mildly basic conditions can be used to achieve the reversible immobilization of the ALP enzyme by denaturing the DNA. The removal of the ALP-cDNA conjugates from the surface of the MNPs was confirmed by a reduction or complete loss in the enzymatic activity of the material (less than 3%) after a mild rinse (Fig. 5b). Furthermore, sequential hybridization and dehybridization processes were performed to confirm the reversibility of the enzyme anchoring process and the capacity for its recovery and reuse. The ALP-MNPs retained above 69.8% residual activity after 5 hybridizations. The results of these tests clearly showed that the DDI method exhibited high reversibility and reproducibility.3.5. Inhibition assay of anchored ALP
The abnormal activity and over-expression of ALP can be regarded as non-specific markers of the presence of a tumor. Several other physiological abnormalities can also be indicated by elevated levels of ALP. The best conditions for screening potential inhibitors of a given enzyme are physiological conditions with the enzyme in free solution. Enzymes immobilized onto MNPs could be used to simulate free enzymes for further study. In this study, theophylline and L-phenylalanine (L-Phe) were selected as inhibitors to evaluate the function of ALP-DNA-MNPs.Compared with an experiment conducted in the absence of an inhibitor (Scheme 1, inset a), the addition of theophylline led to a salient loss in the peak area of the product (Scheme 1, inset b). The Michaelis-constant (Km) is an important kinetic constant, and this value was determined using the Lineweaver–Burk strategy to investigate the bioactivity of the immobilized ALP.38 The Km value of the ALP-DNA-MNPs was determined to be 94.62 μM. This value was similar to a previously reported literature value.39,40 The inhibitor plots for theophylline and L-phenylalanine were established at different inhibitor concentrations (whilst keeping the concentration of 4-NPP at the same level), as shown in Fig. 6. The resulting IC50 values of theophylline and L-Phe were determined to be 0.283 and 8.91 mM, respectively. The IC50 value of theophylline was much smaller than that of L-Phe, which revealed that the inhibitory activity of theophylline was much stronger than that of L-Phe. The Ki (inhibition constant) values of the immobilized ALP were determined in the presence of the two inhibitors, with theophylline and L-Phe giving IC50 values of 0.326 and 6.28 mM, respectively, which were similar to those of the free enzyme.41,42 This result revealed that the MNP-anchored enzyme provided significant stability for ALP in the presence of theophylline and L-phenylalanine. The Lineweaver–Burk plots of the different inhibitors were obtained at various concentrations (Fig. 7). The resulting plots showed that theophylline exhibited non-competitive inhibition behaviour, whilst L-phenylalanine was an uncompetitive inhibitor. Notably, these results were consistent with the general conclusions of the literature29,40 and demonstrated that the ALP-DNA-MNPs prepared in the current study could be used for the screening and identification of enzyme inhibitors.
4. Conclusions
In this work, we have developed a DNA-directed immobilization technique for the preparation of ALP-functionalized MNPs for use in enzymatic and inhibition assays. The DDI method performed effectively as a mild and efficient approach for the specific and reversible immobilization of enzymes. The anchoring of ALP onto MNPs using the DDI method was confirmed by confocal laser scanning microscopy and several other characterization methods. The strand length of the DNA affected the enzymolysis efficiency of the ALP-DNA-MNPs, with a strand length of 24 bases providing the optimal results. Furthermore, the ALP-DNA-MNPs exhibited good enzymolysis and high repeatability properties for enzymatic hydrolysis and inhibition studies, which could be analyzed by HPLC. For the immobilized enzyme, the DDI technique could be used to develop a common method for the reversible and self-directed immobilization of enzymes, which could allow for the regeneration of the enzyme and surface of the carrier for sustainable immobilization of different single enzyme system and multi-enzyme system. Moreover, this method could be extended to the anchoring of a variety of different enzymes and could therefore be used for the high-throughput screening of inhibitors.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 21075008), the Beijing Natural Science Foundation (Grant No. 2132048) and the Fundamental Research Funds for the Central Universities (Grant No. JD1516).Notes and references
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
- P. Zucca and E. Sanjust, Molecules, 2014, 19, 14139–14194 CrossRef PubMed.
- Y. Jiang, Y. Wang, H. Wang, L. Zhou, J. Gao, Y. Zhang, X. Zhang, X. Wang and J. Li, New J. Chem., 2015, 39, 978–984 RSC.
- F. Zhao, H. Li, Y. Jiang, X. Wang and X. Mu, Green Chem., 2014, 16, 2558 RSC.
- E. Magner, Chem. Soc. Rev., 2013, 42, 6213–6222 RSC.
- I. Serra, S. Daly, A. R. Alcantara, D. Bianchi, M. Terreni and D. Ubiali, RSC Adv., 2015, 5, 23569–23577 RSC.
- T. B. Goriushkina, A. P. Soldatkin and S. V. Dzyadevych, J. Agric. Food Chem., 2009, 57, 6528–6535 CrossRef CAS PubMed.
- H. Zhang, R. Liu and J. Zheng, Analyst, 2012, 137, 5363–5367 RSC.
- N. Chauhan and C. S. Pundir, Anal. Methods, 2011, 3, 1360–1365 RSC.
- P. Laveille, A. Falcimaigne, F. Chamouleau, G. Renard, J. Drone, F. Fajula, S. Pulvin, D. Thomas, C. Bailly and A. Galarneau, New J. Chem., 2010, 34, 2153–2165 RSC.
- S. Wang, H. Fang, Y. Wen, M. Cai, W. Liu, S. He and X. Xu, RSC Adv., 2015, 5, 57286–57292 RSC.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Biotechnol. Adv., 2012, 30, 489–511 CrossRef CAS PubMed.
- F. Bano, L. Fruk, B. Sanavio, M. Glettenberg, L. Casalis, C. M. Niemeyer and G. Scoles, Nano Lett., 2009, 9, 2614–2618 CrossRef CAS PubMed.
- G. Arrabito, S. Reisewitz, L. Dehmelt, P. I. Bastiaens, B. Pignataro, H. Schroeder and C. M. Niemeyer, Small, 2013, 9, 4243–4249 CrossRef CAS PubMed.
- Y. Jung, J. M. Lee, H. Jung and B. H. Chung, Anal. Chem., 2007, 79, 6534–6541 CrossRef CAS PubMed.
- Z. Yang, B. Kasprzyk-Hordern, S. Goggins, C. G. Frost and P. Estrela, Analyst, 2015, 140, 2628–2633 RSC.
- L. Du, L. Zou, Q. Wang, L. Zhao, L. Huang, P. Wang and C. Wu, Sens. Actuators, B, 2014, 217, 186–192 CrossRef.
- E. S. Douglas, R. A. Chandra, C. R. Bertozzi, R. A. Mathies and M. B. Francis, Lab Chip, 2007, 7, 1442–1448 RSC.
- N. C. Seeman, Nature, 2003, 421, 427–431 CrossRef PubMed.
- C. Lin, Y. Liu, S. Rinker and H. Yan, ChemPhysChem, 2006, 7, 1641–1647 CrossRef CAS PubMed.
- C. M. Niemeyer, Trends Biotechnol., 2002, 20, 395–401 CrossRef CAS PubMed.
- C. M. Niemeyer, Angew. Chem., Int. Ed. Engl., 2010, 49, 1200–1216 CrossRef CAS PubMed.
- L. Fruk, J. Müller, G. Weber, A. Narváez, E. Domínguez and C. M. Niemeyer, Chem.–Eur. J., 2007, 13, 5223–5231 CrossRef CAS PubMed.
- C. Boozer, J. Ladd, S. Chen, Q. Yu, J. Homola and S. Jiang, Anal. Chem., 2004, 76, 6967–6972 CrossRef CAS PubMed.
- X. Liu and G. Shen, New J. Chem., 2015, 39, 6965–6969 RSC.
- L. Fruk, J. Kuhlmann and C. M. Niemeyer, Chem. Commun., 2009, 230–232 RSC.
- T. D. Chung, E. Sergienko and J. L. Millan, Molecules, 2010, 15, 3010–3037 CrossRef CAS PubMed.
- D. Ghosh, C. N. Roy, S. Mondal, S. Kundu, S. Maiti, P. K. Bag and A. Saha, RSC Adv., 2016, 6, 5024–5031 RSC.
- B. A. Zaccheo and R. M. Crooks, Langmuir, 2011, 27, 11591–11596 CrossRef CAS PubMed.
- Y. Li, Y. Li, X. Wang and X. Su, New J. Chem., 2014, 38, 4574–4579 RSC.
- J. Huang, P. Su, J. Wu and Y. Yang, RSC Adv., 2014, 4, 58514–58521 RSC.
- D. Hong, L. Xiaolin, P. Qing, W. Xun, C. Jinping and L. Yadong, Angew. Chem., 2005, 117, 2842–2845 CrossRef.
- Y. Deng, D. Qi, C. Deng, X. Zhang and D. Zhao, J. Am. Chem. Soc., 2008, 130, 28–29 CrossRef CAS PubMed.
- Y. Xiang and Y. Lu, Anal. Chem., 2012, 84, 1975–1980 CrossRef CAS PubMed.
- E. Akyilmaz and M. Turemis, Electrochim. Acta, 2010, 55, 5195–5199 CrossRef CAS.
- A. Singh, S. Snyder, L. Lee, A. P. Johnston, F. Caruso and Y. G. Yingling, Langmuir, 2010, 26, 17339–17347 CrossRef CAS PubMed.
- M. Mazorra, J. Rubio and J. Blasco, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2002, 131, 241–249 CrossRef CAS.
- J. Saevels, K. Van den Steen, A. Van Schepdael and J. Hoogmartens, J. Chromatogr. A, 1996, 745, 293–298 CrossRef CAS.
- J. Iqbal, Anal. Biochem., 2011, 414, 226–231 CrossRef CAS PubMed.
- S. Wang, P. Su, J. Huang, J. Wu and Y. Yang, J. Mater. Chem. B, 2013, 1, 1749 RSC.
- A. R. Whisnant and S. D. Gilman, Anal. Biochem., 2002, 307, 226–234 CrossRef CAS PubMed.
- K. W. Gasser and L. B. Kirschner, J. Comp. Physiol., B, 1987, 157, 461–467 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the DNA sequences; characterization techniques- TGA curves and magnetization curves. See DOI: 10.1039/c6ra01621a |
| This journal is © The Royal Society of Chemistry 2016 |






