Review on fungal enzyme inhibitors – potential drug targets to manage human fungal infections
Abstract
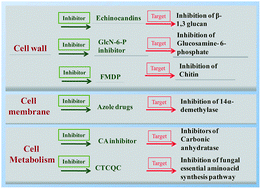
Invasive fungal infections caused by opportunistic fungal pathogens have been emerging as a global problem of great concern as they are associated with increased morbidity and mortality. Despite this, there are very limited drugs of choice to treat fungal infections. The continuous usage of these drugs is associated with resistance development and thus this is another area of concern. Fungal enzymes represent one of the most important and potential targets for drug development, as they are essential for their growth and establishment in the host. In this review, we have discussed the well established and currently available enzyme inhibitors as therapeutic choices to treat fungal infections as well as those enzyme inhibitors that have been identified as suitable drug candidates to manage fungal infections. Thus, the study of fungal biosynthetic enzymes and their inhibitors could potentially show a promising way of drug development for emerging and re-emerging fungal infections of humans.

 Please wait while we load your content...
Please wait while we load your content...