Dual-functional α-NaYb(Mn)F4:Er3+@NaLuF4 nanocrystals with highly enhanced red upconversion luminescence†
Abstract
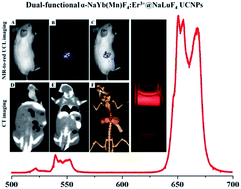
Rare earth doped upconversion nanoparticles (UCNPs) used as bioprobes for in vivo upconversion luminescence (UCL) deep tissue imaging have been extensively studied. Red emission possesses high penetration length in biological tissue, so it's more convenient for naked eyed red emission to be performed in biological imaging and detection. However, improved in vivo imaging depth based on the NIR-to-red UCL is still challenging and desirable. Herein, novel α-NaYb(Mn)F4:Er3+@NaLuF4 core–shell nanocrystals with enhanced red emission and improved imaging depth have been synthesized. Under 980 nm excitation, there is a 220% increase in red-to-green ratio and even a 400% increase in red-emission when the Yb3+ core doping concentration is increased from 18% to 68% in the core–shell structure. And the UCL signals detection of pork muscle tissues could be visualized even at a depth of 18 mm. By the tail vein injection of PEG modified α-NaYb(Mn)F4:Er3+@NaLuF4 UCNPs, the in vivo and ex vivo NIR-to-red UCL deep tissue imaging present significant UCL signals in liver and spleen. Moreover, the UCNPs could also be used as in vitro and in vivo X-ray computed tomography (CT) contrast agent owing to the large X-ray absorption efficiency and high atomic number of ytterbium and lutetium elements. The synergistic combination of the in vivo NIR-to-red UCL deep tissue and CT imaging could provide comprehensive diagnosis information for cancer.

 Please wait while we load your content...
Please wait while we load your content...