A new lipase–inorganic hybrid nanoflower with enhanced enzyme activity
Abstract
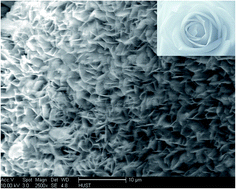
A new hybrid nanoflower was synthesized using the organic component of Burkholderia cepacia lipase and inorganic component of calcium phosphate. Under the optimum conditions, the nanobiocatalyst exhibited an activity of 849.8 U, 308% folds of the free one, with relatively good stability, highlighting its potential for industrialization.

 Please wait while we load your content...
Please wait while we load your content...