Well-defined degradable brush-coil block copolymers for intelligent release of insulin at physiological pH†
Xuan Zhanga,
Liyuan Zhaoa,
Junjiao Yangb and
Jing Yang*a
aState Key Laboratory of Chemical Resource, College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: yangj@mail.buct.edu.cn; Fax: +86-10-64427578; Tel: +86-10-64427578
bCollege of Science, Beijing University of Chemical Technology, Beijing 100029, China
First published on 17th February 2016
Abstract
To achieve an intelligent glucose responsive insulin delivery system with reasonable glucose-sensitivity and minimal long-term side effects, a kind of degradable brush amphiphilic polymer was synthesized through grafting-from strategy of poly[(2-phenylborate esters-1,3-dioxane-5-ethyl) methylacrylate] (PPBDMMA) as a side chain via atom transfer radical polymerization (ATRP) and the poly(ε-caprolactone) (PCL) backbone fabricated by ring-opening polymerization of α-bromo-caprolactone (α-BrCL) monomers using monomethylpoly(ethylene glycol) (MPEG) as an initiator. The well-controlled structures of the resulting brush polymers were verified by 1H NMR and gel permeation chromatography (GPC) characterizations. These brush polymers MPEG-b-P(CL-g-PPBDMMA) were self-assembled to form polymeric micelles with a hydrophobic core composed of both glucose-responsive PPBDMMA and biodegradable hydrophobic PCL. These nanocarriers exhibited a very low insulin release at a glucose concentration of 1.0 mg mL−1 (normoglycemia) and a relatively rapid release at 3.0 mg mL−1 (hyperglycemia) at pH 7.4. The glucose-triggered on–off release of insulin at pH 7.4 with alternate 1.0 and 3.0 mg mL−1 glucose incubation further exhibited effective controllable insulin delivery in response to physiological glucose level fluctuation. The cell viability of all the nanoparticles investigated by the MTS assay was higher than 80%, indicating that these brush polymers had good cytocompatibility. The benign degradability of these nanocarriers in the presence of Novozym 435 was evidenced in this study. This type of nanocarrier may be a promising candidate for in vivo insulin delivery.
Introduction
Molecular brushes are an interesting macromolecular architecture comprised of a linear backbone and densely grafted side chains.1–4 The backbones of molecular brushes adopt an extended conformation resulting from steric repulsions between the side chains, and the highly congested and constrained structure affords distinctive properties which cannot be easily achieved in other systems.5–8 Due to their unique properties, molecular brushes have gained increasing attention for potential applications that range from nanotechnology9,10 to biomedical fields.11–13 In recent years, great efforts have been contributed to bringing stimuli-responsiveness to molecular brushes. The combination of the unique architecture of brush polymers with stimuli-responsive properties provides a great opportunity to develop intelligent materials.14–21Insulin-dependent diabetes is a serious disease severely impinging on quality of life, and even threatens the life.22,23 Great efforts are devoted to exploring glucose-responsive materials for controlled closed-loop insulin delivery systems, which can mimic pancreatic function, detect elevated glucose levels, and automatically “secrete” insulin in response to the blood glucose levels.24–26 Two critical issues, effective glucose-responsiveness at physiological pH and reasonable glucose-sensitivity (1–3 mg mL−1), are demanded for one ideal glucose responsive insulin delivery system (GRIDS). Phenylboronic acid (PBA) and its derivatives are one class of the most attracting glucose-responsive systems, due to their versatility for different designs and better stability.27–32 Since the pioneer work by Kataoka to decrease the apparent pKa of phenylboronic acid (PBA),33–35 significant progress has been achieved on constructing a variety of PBA-based glucose-responsive materials with an appreciably glucose-responsive behavior at pH 7.4.36–47 In recent years, polymeric micelles, vesicles, and microgels formed from PBA-based materials are well developed to exhibit smart glucose-responsiveness under the physiological and pathologic condition.48–51 Notably, the majority of these reported works intend to design linear polymers based on PBA. Introducing special molecular architectures into glucose-responsive materials for insulin intelligent administration to match physiological needs still remains a challenge.
From the biomedical perspective, materials with better biodegradability and biocompatibility are favoured as alternatives. Whereas, there are only a few reports on the biodegradable PBA derivatives with glucose-responsiveness,52–56 although intensive studies on nondegradable poly(acrylic acid) based PBA-containing polymers32–34 have been reported, which are unfavourable for blood clearance after insulin deliver and may cause long-term toxicity. Recently, polypeptide strategies have been developed to incorporate degradability with the PBA-based drug delivery systems. For example, Chen and coworkers reported glucose-sensitive polypeptide micelles formed by monomethoxy poly(ethylene glycol)-b-poly(L-glutamic acid-co-N-3-L-glutamylamidophenyl boronic acid) (mPEG-b-P(GA-co-GPBA)) with better biocompatibility for self-regulated insulin release at physiological pH.52 Shi's group successfully developed a biodegradable and glucose-responsive complex polymeric micelles through the self-assembly of poly(aspartic acid)-containing amphiphilic diblock copolymers, which exhibited a reversible swelling, enabling the repeated on–off release of insulin regulated by the glucose level.53,54 Very recently, this group further described a kind of glucose-responsive polymer vesicles via poly(aspartic acid)-containing glycopolymer mixing with PBA-containing thermo-sensitive amphiphilic polymers,55,56 which was favorable for the penetration of water-soluble substances and exhibited prominent glucose-responsiveness at physiological pH 7.4. Besides polypeptide, aliphatic polyesters, as an important class of biodegradable and biocompatible polymers, have been broadly explored for therapeutic delivery applications.57–59 In most cases, aliphatic polyesters were suitably used for drug encapsulation via nanoparticles or polymeric micelles,60,61 because of their feasible preparation in comparison with polypeptide. However, studies on aliphatic polyester-based stimuli-responsive materials were rather limited, mainly due to the lack of functionality.
We previously developed the polymers containing pinacol boronate ester structure for insulin intelligent administration at neutral pH based on the combination of the competition mechanism between sugar molecule and acyclic diol with the characteristics of boronate ester with low pKa.41,42,51 Recently, we imparted biodegradable poly(lactic acid) PLA into such glucose-responsive polymer architecture and synthesized a kind of Y-shaped block polymers poly(ethylene glycol)-block-[poly(lactic acid)-block-poly(2-phenylborate esters-1,3-dioxane-5-methyl) methylacrylate]2 MPEG-(PLA-block-PPBDMMA)2 with PPBDMMA moieties at ω-terminals of PLAs via a combination of ring-opening polymerization (ROP) and atom transfer radical polymerization (ATRP).43 Although the introduction of PLA endowed the whole Y-shaped polymers with good biodegradability and strong hydrophobic driving-force for the self-assembling of the polymers in aqueous solution, these nanocarriers from MPEG-(PLA-block-PPBDMMA)2 exhibited unsatisfied responsive difference between normoglycemia and hyperglycemia, which stays away from the purpose of self-regulated delivery.
In order to achieve glucose-responsive materials with minimal long-term side effects for controlled closed-loop insulin delivery, a new class of GRIDS, biodegradable brush amphiphilic polymers, is explored in this study. Such phenylborate esters-containing brush polymers poly(ethylene glycol)-block-poly[(ε-caprolactone)-graft-poly[(2-phenylborate esters-1,3-dioxane-5-methyl) methylacrylate] MPEG-b-P(CL-g-PPBDMMA) were synthesized through PPBDMMA segments via ATRP grafting from the PCL backbone which was prepared by ring-opening polymerization of α-bromo-caprolactone monomers using MPEG as initiator. The design of brush architecture bearing PPBDMMA as side chains endowed the resulting polymers with highly dense glucose responsive groups, exceeding the synthesis limitation of linear structure under identical polymerization condition. The introduction of interesting molecule structure would strengthen the hydrophobic driving force of the whole polymers, benefiting to the formation of stable nanoparticle in aqueous environment, and avoiding the immediate collapse of the nanocarriers in response to glucose, which would be dedicated to enhancing the glucose concentration selectivity and realizing controllable insulin release. With PCL backbone degrading in the presence of lipase, the brush polymers would be dispersed into fragments such as oligomers, significantly avoiding long-term toxicity of the delivery system to body.
Experimental section
Materials
Pentamethyl diethylenetriamine (PMDETA, 99%) from Acros Organics was used without further purification. CuBr purchased from Aldrich was purified by stirring in acetic acid overnight, followed by washing with ethanol and diethyl ether, and dried in vacuum. Cyclohexanone and Sn(Oct)2 from Aldrich were dried with CaH2 for overnight, followed by distillation in vacuum, respectively. Monomethylpoly(ethylene glycol) MPEG 5000 (Mn = 5000) purchased from Aldrich was dealt with azeotropic dehydration with anhydrous toluene prior to use. Insulin (27 UI mg−1) purchased from Genview was labeled by fluorescein isothiocyanate (FITC) based on the previous report.62 Monomer (2-phenylborate esters-1,3-dioxane-5-methyl) methylacrylate (PBDMMA) was prepared according to the reported methods.41 Toluene and anisole were dried with sodium using benzophenone as an indicator. Triethylamine (TEA) and methylene dichloride (CH2Cl2) were dehydrated with KOH and CaCl2 overnight and distilled, respectively. The immobilized Novozym 435 lipase (with a nominal activity of 10 U mg−1) was obtained from Beijing Puboxin Biochemical Co. Ltd. (Beijing, China). NIH 3T3 mouse broblast cells were provided from Beijing Xiehe Cell Resource Center. DMEM and MTS assay kit were purchased from Beijing Promega Biochemical Co. Ltd. (Beijing, China). All other reagents were of analytical grade and were used without further purification. Synthesis of α-brominated caprolactone (α-BrCL) was described in ESI.†Characterization
The chemical structures of the copolymers were characterized by 1H and 13C NMR carrying out on a 400 MHz NMR instrument (Bruker Corporation, Germany) at room temperature using CDCl3 as solvent. The chemical shifts were measured against the solvent signal of CDCl3 as internal standard. The molecular weights and polydispersity index of the polymers were determined with Waters 515-2410 gel permeation chromatograph (GPC) instrument equipped with Styragel HT6E-HT5-HT3 chromatographic column following a guard column and a differential refractive-index detector. The measurements were performed using THF as eluent (flow rate of 1.0 mL min−1 at 30 °C) and a series of narrowly distributed polystyrene standards for the calibration. The fluorescence spectra were recorded by a Hitachi F-4600 Fluorescence instrument (Hitachi High-Technologies Corporation, Tokyo Japan) at 37 °C. The particle size and zeta potential of FITC–insulin loading and unloading nanoparticles were both measured with Nano-ZS (ZEN3600, Malvern) equipped with Zetasizer software and with 35 mW solid state laser operated at a laser light wavelength of 660 nm. The size measurements were carried out at 25 °C at a scattering angle of 90°. The nanoparticles were imaged on a Hitachi H800 transmission electron microscopy (TEM) (Hitachi High-Technologies Corporation, Tokyo, Japan) operated with 100 kV.Synthesis of poly(ethylene glycol)-block-poly(α-brominated caprolactone) (MPEG-b-PBrCL)
To realize self-assembly of the brush amphiphilic polymers in aqueous solution without aid of surfactant, MPEG 5000 was selected as hydrophilic block in this study. MPEG 5000 (0.75 g, 0.15 mmol) and α-BrCL (1.0 g, 5.2 mmol) placed in a dried polymerization tube were dissolved in dried toluene (1.0 mL). After the tube was subjected to five vacuum–nitrogen atmosphere cycles, Sn(Oct)2 (60.0 mg, 0.15 mmol) as the catalyst was added by syringe. The mixture was stirred at 90 °C for 4 h, then precipitated in cold diethyl ether for four times to obtain white solid MPEG-b-PBrCL in a yield of 60%. 1H NMR (400 MHz, CDCl3), δ (ppm): 4.20 (m, 3H, –COOCH2–, –OCOCH(Br)), 3.46–3.79 (m, 2H, –CH2–CH2–), 3.37 (s, 3H, –OCH3), 2.06 (m, 2H, –CH(Br)CH2–), 1.71 (m, 2H), 1.45–1.58 (m, 2H, –CH2CH2CH2OOC–); 13C NMR (400 MHz, CDCl3), δ (ppm): 169.67, 72.16, 70.82, 68.85, 65.49, 62.38, 59.10, 45.70, 34.37, 31.92, 27.80, 23.81.Synthesis of poly(ethylene glycol)-block-poly[(ε-caprolactone)-graft-poly[(2-phenyl borate esters-1,3-dioxane-5-methyl) methylacrylate] MPEG-b-P(CL-g-PPBDMMA)
MPEG-b-P(CL-g-PPBDMMA) was synthesized by ATRP polymerization of different amount of PBDMMA monomers using MPEG-b-PBrCL as macroinitiator. The synthesis of MPEG-b-P(CL-g-PPBDMMA5)25 as an example was described. MPEG-b-PBrCL (98.0 mg, 0.01 mmol), PBDMMA (1.37 g, 5.0 mmol), CuBr2 (3.35 mg, 0.015 mmol), PMEDTA (51.9 mg, 0.3 mmol) and 2.0 mL anisole were added into a Schlenk tube, which was subjected to three freeze–pump–thaw cycles. CuBr (43.0 mg, 0.3 mmol) was subsequently added under nitrogen atmosphere. After the mixture was stirred at 100 °C for 24 h, the catalyst was removed through a neutral alumina column and the concentrated polymerization solution was precipitated into an excess of a n-hexane and diethyl ether mixture (v/v = 5/1) for three times to afford white polymer in a yield of 30.8%. 1H NMR (400 MHz, CDCl3), δ (ppm): 7.75 (d, 2H, o-C6H5), 7.38 (m, 1H, p-C6H5), 7.30 (m, 2H, m-C6H5), 3.68–4.05 (m, 6H, –BOCH2CCH3, –BOCH2CCH2O–, –COOCH2–), 3.65 (m, –OCH2CH2–), 2.30 (1H, –CHCH2– and OCCHCH2), 1.44–1.92 (m, 2H, –CH2 from PCL backbone and acrylate structure), 0.86 (s, 3H, –CH3 from PBDMMA); 13C NMR (400 MHz, CDCl3), δ (ppm): 173.74, 133.69, 131.64, 127.36, 70.33, 67.70, 65.30, 35.15, 31.48, 22.41, 17.42, 13.88.Other polymerization condition was kept unchanged, and only the amount of monomer PBDMMA was changed to 2.05 g (7.5 mmol) and 2.75 g (10.0 mmol) to prepare MPEG-b-P(CL-g-PPBDMMA8)25 and MPEG-b-P(CL-g-PPBDMMA10)25. Their yields were 35.8% and 39.2%, respectively.
Preparation of the nanoparticles from MPEG-b-P(CL-g-PPBDMMA)
Briefly, to 10.0 mg MPEG-b-P(CL-g-PPBDMMA) dissolved in 0.5 mL THF was added ultrapure water (10 mL) at a rate of one drop (ca. 0.02 mL) per second under vigorous stirring in ice bath. After 12 h, the solution was transferred into dialysis membrane (molecular weight cut off 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and dialyzed for 48 h against ice ultrapure water to remove the organic solvent, and ultrapure water was refreshed every 6 h. The morphology of the nanoparticles was measured by TEM and DLS, respectively.
000) and dialyzed for 48 h against ice ultrapure water to remove the organic solvent, and ultrapure water was refreshed every 6 h. The morphology of the nanoparticles was measured by TEM and DLS, respectively.
Evaluation of insulin-loading capacity of the nanoparticles
As example, a stock of FITC–insulin (3.0 mg) dissolved in 10.0 mL HCl (0.01 M) was adjusted to pH 6.0 using NaOH (0.1 M), and dropwise added into the polymer (10.0 mg) in 0.5 mL THF with strong stirring in ice bath overnight. The purification of the nanoparticles encapsulating FITC–insulin was performed by dialysis method (MWCO, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), and monitored by fluorescence technique.
000 Da), and monitored by fluorescence technique.
The entrapment efficiency of insulin was determined using a fluorescence instrument after the isolation of free FITC–insulin from the micelle solution via the above-mentioned purification method. The encapsulated insulin mass was calibrated according to the measured standard curve of fluorescence intensity against insulin concentration. The insulin entrapment efficiency (EE) and loading capacity (LC) were calculated using the following equations:
Responsive release of FITC–insulin
The in vitro release test of FITC–insulin from the nanoparticles was evaluated by the dialysis method. The insulin-loaded solution was sealed by a dialysis membrane (MWCO 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and immersed into PBS (0.01 M, pH 7.4) at 37 °C with different glucose concentrations (0, 1.0 and 3.0 mg mL−1) while shaking (100 rpm). 1.0 mL of the external medium was sampled every determined time. The emission intensity was measured by fluorescence spectroscopy at an emission wavelength of 525 nm upon excitation at 494 nm. The cumulative release percentage at determined time was calculated by the ratio of the fluorescence intensity at that time to the sum of the released intensity.
000) and immersed into PBS (0.01 M, pH 7.4) at 37 °C with different glucose concentrations (0, 1.0 and 3.0 mg mL−1) while shaking (100 rpm). 1.0 mL of the external medium was sampled every determined time. The emission intensity was measured by fluorescence spectroscopy at an emission wavelength of 525 nm upon excitation at 494 nm. The cumulative release percentage at determined time was calculated by the ratio of the fluorescence intensity at that time to the sum of the released intensity.
Circular dichroism spectroscopy
The stability of the released insulin was determined by analysis of the conformation of released insulin using circular dichroism (CD), and the resulting spectrum was compared to standard insulin. The standard insulin solution was prepared in PBS (0.01 M, pH 7.4) to a final concentration of 30 μg mL−1. CD measurements were performed on a Jasco J-810 CD spectropolarimeter at 25 °C with a cell length of 1.0 cm. For the far-UV CD spectra, samples were scanned from 190 to 260 nm and accumulated 5 times, at a resolution of 1.0 nm and scanning speed of 700 nm min−1.In vitro cytotoxicity of the nanoparticles
The in vitro cytotoxicity was evaluated by MTS assay. NIH 3T3 mouse broblast cells were seeded into 96-well plates at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and incubated for 24 h. The prepared nanoparticle solutions were diluted to give a range of final concentration from 12.5 to 500 mg L−1 using DMEM (Dulbecco's Modified Eagle Medium) solutions as the culture medium. The plates were maintained in the incubator for 24, 48 and 72 h, respectively. After incubation, 20 μL of MTS solution was added to each well. Four hours later, the optical density of the solution was measured at 492 nm using a microplate reader (Labsystem, Multiskan, Ascent, Finland).
000 cells per well and incubated for 24 h. The prepared nanoparticle solutions were diluted to give a range of final concentration from 12.5 to 500 mg L−1 using DMEM (Dulbecco's Modified Eagle Medium) solutions as the culture medium. The plates were maintained in the incubator for 24, 48 and 72 h, respectively. After incubation, 20 μL of MTS solution was added to each well. Four hours later, the optical density of the solution was measured at 492 nm using a microplate reader (Labsystem, Multiskan, Ascent, Finland).
In vitro enzymatic degradation of the nanoparticles
The in vitro degradation of the nanoparticles (1.0 mg mL−1) was performed at 37 °C in the presence of the immobilized Novozym 435 lipase (0.1 mg mL−1) in ultrapure water at pH 7.4. The release of the encapsulated FITC–insulin from the micelles was detected to estimate enzymatic degradation behavior. The degraded samples were taken out at predetermined time intervals and dialyzed against water (MWCO 3500) for 48 h. After lyophilization, chemical structure was determined by 1H NMR.Results and discussion
Synthesis and characterization of MPEG-b-P(CL-g-PPBDMMA)
Based on the good biodegradability of PCL, herein, the amphiphilic brush polymers were designed with MPEG as hydrophilic block, and the hydrophobic block comprising of biodegradable PCL as backbone and glucose responsive PPBDMMA as side chains. These brush polymers MPEG-b-P(CL-g-PPBDMMA) were fabricated by grafting-from synthesis via incorporation of ROP and ATRP, as shown in Scheme 1. Accordingly, α-bromo-caprolactone (α-BrCL) needs to be prepared first for the construction of PCL backbone (see ESI†). Using α-BrCL as monomer, MPEG as initiator and Sn(Oct)2 as catalyst ([α-BrCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [MPEG]
[MPEG]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Sn(Oct)2] = 35
[Sn(Oct)2] = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
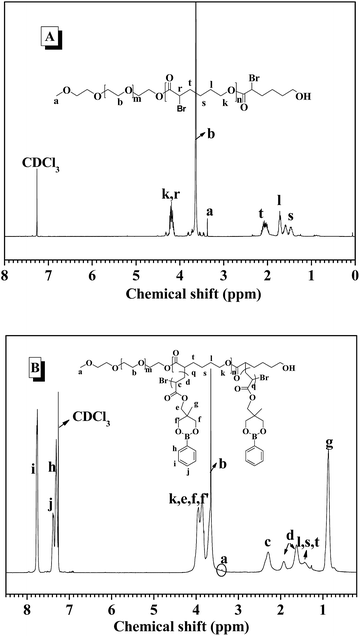
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the amphiphilic block polymer MPEG-b-PBrCL was obtained by ROP in toluene at 90 °C for 4 h. After precipitation of the polymerization solution in cold methanol, the resulting MPEG-b-PBrCL was analyzed by 1H NMR technique (Fig. 1A) and GPC (Fig. S7†), and its well-controlled chemical structure was verified. The number-average degree of polymerization (DPn) of PBrCL block was determined as 25 by comparing the resonance intensities of the protons from the methine and methylene of PCL block (denoted as r and k, respectively) around 4.20 ppm with those of the terminal CH3 protons from MPEG at 3.37 ppm, as shown in Fig. 1A. According to the DPNMRn value, the number-average molecular weight (Mn) of the polymer was further determined as 9800, similarly to Mn of 9000 characterized by GPC analysis. PDI of 1.23 relative to linear polystyrenes indicated the good synthetic control in the polymerization.
1), the amphiphilic block polymer MPEG-b-PBrCL was obtained by ROP in toluene at 90 °C for 4 h. After precipitation of the polymerization solution in cold methanol, the resulting MPEG-b-PBrCL was analyzed by 1H NMR technique (Fig. 1A) and GPC (Fig. S7†), and its well-controlled chemical structure was verified. The number-average degree of polymerization (DPn) of PBrCL block was determined as 25 by comparing the resonance intensities of the protons from the methine and methylene of PCL block (denoted as r and k, respectively) around 4.20 ppm with those of the terminal CH3 protons from MPEG at 3.37 ppm, as shown in Fig. 1A. According to the DPNMRn value, the number-average molecular weight (Mn) of the polymer was further determined as 9800, similarly to Mn of 9000 characterized by GPC analysis. PDI of 1.23 relative to linear polystyrenes indicated the good synthetic control in the polymerization.
 | ||
| Fig. 1 1H NMR spectra of macroinitiator MPEG-b-PBrCL (A); the brush polymer MPEG-b-P(CL-g-PPBDMMA) (B). | ||
MPEG-b-PBrCL not only provides biodegradable hydrophobic main chain, but also endows with the opportunity of constructing brush structure due to the presence of α-Br group in each repeating unit of PCL backbone. Subsequently, the graft-from synthesis was conducted by using MPEG-b-PBrCL as macroinitiator to initiate PBDMMA monomers via ATRP in anisole at 100 °C for 24 h (Scheme 1). By adjusting polymerization condition, such as changing the feeding molar ratio of repeating unit BrCL to monomer PBDMMA, three distinct brush copolymers having different PPBDMMA content were obtained, as listed in Table 1. The chemical structure of brush polymers MPEG-b-P(CL-g-PPBDMMA) was confirmed by 1H NMR analysis (Fig. 1B). Besides that the characteristic signals (a, b, s, t) from MPEG and PCL main chain were observed at the corresponding sites, the peaks around 3.90 and 0.86 ppm were assigned to the protons of the ester methylene (e), oxymethylene (f, f′) and methyl group (g) in PPBDMMA block. The characteristic resonances of phenyl protons (i, j, h) were identified between 7.28 and 7.80 ppm. The chemical components of the PPBDMMA blocks were estimated by comparing to the peak integral ratio between phenyl ring of PBDMMA moiety at 7.76 ppm and the CH2 protons (a) of PCL between 1.0 and 2.0 ppm, suggesting each CL unit bearing average 5–10 PBDMMA repeating units as side chain. The overall molecular weights of the resulting brush polymers calculated based on 1H NMR spectra were listed in Table 1. Furthermore, GPC measurements exhibited unimodal and relatively narrow distribution without MPEG-b-PBrCL (Fig. S7†), revealing the successful synthesis of structurally well-defined brush polymers.
| Sample | Polymerc | Initiator | [I]/[M] | Mnf (NMR) | GPCg | |
|---|---|---|---|---|---|---|
| Mn | PDI | |||||
| a MI represented the macroinitiator MPEG-b-PBrCL.b BP indicated brush polymer MPEG-b-P(CL-g-PPBDMMA).c The numbers at the footnote showing the average repeating unit number of each moiety were determined by 1H NMR; the number at the footnote of PPBDMMA indicated the grafting number on each CL unit; 25 represented the repeating unit number of hydrophobic main chain.d Indicating molar ratio of MPEG to monomer α-BrCL.e Meaning the molar ratio of Br of BrCL repeating units to PBDMMA monomers.f The number-average molecular weights of the block copolymers were calculated by 1H NMR results.g Determined by GPC. | ||||||
| MIa | MPEG-b-PBrCL25 | MPEG 5000 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35d 35d |
9800 | 9000 | 1.23 |
| BP-1b | MPEG-b-P(CL-g-PPBDMMA5)25 | MI | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20e 20e |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.26 |
| BP-2b | MPEG-b-P(CL-g-PPBDMMA8)25 | MI | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30e 30e |
65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.24 |
| BP-3b | MPEG-b-P(CL-g-PPBDMMA10)25 | MI | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40e 40e |
79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.30 |
Self-assembly of the brush polymers MPEG-b-P(CL-g-PPBDMMA)
The amphiphilic brush polymers MPEG-b-P(CL-g-PPBDMMA) consist of soluble MPEG segment in water and insoluble P(CL-g-PPBDMMA) segment in neutral solution, which would self-assemble in aqueous solution. Critical micellar concentration (CMC) was firstly investigated to measure the driving force of these brush polymers in aqueous environment using the reported fluorescence technique (Fig. S8†).63 As listed in Table 2, CMCs of the brush polymers MPEG-b-P(CL-g-PPBDMMA) ranged between 11 and 83 mg L−1, which is lower than our previously reported linear MPEG-b-PPBDMMA series (their CMCs were in the range of 50–120 mg L−1). This indicates that the introduction of brush structure enabled the overall PPBDMMA units number to increase with the side chain extending, strengthened driving force for the self-assembly of the whole copolymers in aqueous solution, beneficial to form stable nanoparticles at relatively low concentration of brush polymers.| Polymer sample | CMC (mg L−1) | Without FITC–insulin | With FITC–insulinc | ||||
|---|---|---|---|---|---|---|---|
| Diametera (nm) | PDI | Zeta potentialb (mV) | Diametera (nm) | PDI | Zeta potentialb (mV) | ||
| a The particle average dimension determined by DLS at 25 °C.b Measured by zeta potential analyzer at 25 °C and pH 7.4.c The applied insulin concentration was 0.3 mg mL−1. | |||||||
| BP-1 | 83 | 147.3 ± 0.89 | 0.292 ± 0.002 | −8.61 | 257.7 ± 0.97 | 0.261 ± 0.036 | −21.9 |
| BP-2 | 25 | 139.5 ± 1.22 | 0.281 ± 0.006 | −10.2 | 240.0 ± 0.98 | 0.373 ± 0.064 | −23.6 |
| BP-3 | 11 | 125.5 ± 0.49 | 0.328 ± 0.001 | −11.5 | 185.1 ± 0.95 | 0.243 ± 0.012 | −26.8 |
The average hydrodynamic diameters of the nanoparticles were measured by DLS. DLS diagram of MPEG-b-P(CL-g-PPBDMMA5)25 was shown in Fig. 2A as an example, and the other results were summarized in Table 2 and Fig. S9.† It is obvious that the average sizes of these nanoparticles were maintained in the range of 120–150 nm. These diameters were slightly decreased with an increase in the PPBDMMA content of the whole hydrophobic moiety. The pristine nanoparticles further characterized by zeta potential at pH 7.4 showed some negative charge, which was probably resulted from the anionic borate ester structure in aqueous solution.64 The particle morphology of the amphiphilic copolymers in the dry state was detected by TEM (Fig. 2B and S10†), and exhibited the closely spherical polymeric micelles with diameter about 100 nm.
 | ||
| Fig. 2 (A) Diameter of nanoparticles from BP-1 without and with insulin; (B) TEM image of the nanoparticles from BP-1 without insulin. | ||
Insulin-loading of the nanoparticles from brush polymers MPEG-b-P(CL-g-PPBDMMA)
Fluorescence-labeled insulin such as FITC–insulin was selected to elucidate the glucose-responsiveness of brush polymers MPEG-b-P(CL-g-PPBDMMA) in this study. By adjusting pH value of the self-assembly aqueous solution to 6.0 close to isoelectronic point of insulin (PI, 5.35–5.45), FITC–insulin was encapsulated in the hydrophobic core of the polymeric micelles MPEG-b-P(CL-g-PPBDMMA) due to the low solubility of insulin in the proximity of pI and the hydrophobic interactions of the polymers. The free insulin in the pristine self-assembled solution was thoroughly removed by multiple ultrafiltration centrifuge (MWCO 30 kDa).42 Subsequently, the insulin-loading polymeric micelles were further characterized by DLS and zeta potential, respectively. As listed in Table 2, comparing to the polymeric micelles without FITC–insulin, the sizes of insulin-loading nanoparticles slightly became bigger, and their zeta potential dramatically decreased to about −20 mV, exhibiting these insulin-loading nanocarriers had better particles' stability in aqueous environment. The interaction between insulin and hydrophobic core of the nanoparticles probably led to size expansion of the polymeric micelles and the surface charge change of the nanocarriers. In addition, no free insulin was detected at −7.2 mV, further substantiating successful encapsulation of insulin in the polymeric micelles and the complete removal of free insulin from the solution.The entrapment efficiency (EE) and loading capacity (LC) of FITC–insulin in the polymeric micelles were examined depending on the hydrophobic PBDMMA content ratio, as shown in Fig. 3. When the polymer concentration was determined at 1.0 mg mL−1 and the given insulin concentration was 0.2 mg mL−1, EE of the polymeric micelles formed from BP-1 with lowest PBDMMA content could reach above 60%, which is greatly higher than the reported linear MPEG-b-PPBDEMA system.42 Moreover, with an increase in PBDMMA content of the brush polymers, EE of FITC–insulin in the polymeric micelles was distinctly enhanced to nearly 74%, and their LC was kept around 17%. The brush architecture with highly dense glucose responsive moieties provided more effective interaction with insulin, contributing to insulin entrapment.
 | ||
| Fig. 3 EE and LC of insulin in the polymeric micelles from BP-1, BP-2 and BP-3 (the given insulin concentration was 0.2 mg mL−1). | ||
Glucose-responsive release of FITC–insulin
In the human body, the blood glucose level is tightly regulated in the range of 0.6–1.2 mg mL−1. When the glucose level exceeds 2.0 mg mL−1, it is generally considered as hyperglycemia, which has to be treated. During exploring intelligent glucose-responsive delivery systems, scientific attention is improved to focus on not only glucose-sensitivity under hyperglycemia conditions but also the real-time response to glucose under normoglycemia (1 mg mL−1 glucose) to avoid a hypoglycemia effect induced by excess insulin released from the drug carriers. Based on the consideration of intelligent demand, the glucose-responsiveness of these brush polymers was evaluated in this study. FITC–insulin-loading nanoparticles were prepared at pH 6.0, and the cumulative release of insulin from these nanoparticles was calculated by monitoring the changes in the fluorescence intensity of the external fluid at pH 7.4. Fig. 4A shows the insulin release profile from these three brush polymers varying hydrophobic block length at varying glucose concentration. The resultant nanoparticles were generally stable in the absence of glucose, and less 10% insulin was released from MPEG-b-P(CL-g-PPBDMMA) in 70 h. When FITC–insulin-loading micelles were incubated at the glucose concentration of 1.0 mg mL−1 (normoglycemic condition), only about 25% insulin release occurred within 70 h measurement. When the glucose concentration was increased to 3.0 mg mL−1 (hyperglycemic condition), the remarkable and sustained insulin release was subsequently observed at a relatively stable release rate. Notably, no burst release occurred within the first 20 hours of glucose treatment, indicating no free insulin or insulin absorbed onto the surface of the nanoparticles after the above purification. Additionally, varying PBDMMA content of the brush polymers also led to slightly different glucose-responsiveness. At the glucose concentration of 3.0 mg mL−1, as PBDMMA content was increased in the hydrophobic blocks, insulin was gradually released from the micelles in the following order: MPEG-b-P(CL-g-PPBDMMA5)25 (BP-1) > MPEG-b-P(CL-g-PPBDMMA8)25 (BP-2) > MPEG-b-P(CL-g-PPBDMMA10)25 (BP-3). Comparing to the brush polymer having higher PBDMMA content, the lower PBDMMA density the brush polymer contains, the easier it is for glucose molecules to penetrate into the hydrophobic core of the nanoparticles and react with phenylborate ester structure of PPBDMMA block, leading to fast insulin release.The change of nanoparticle size was measured by DLS during the process of glucose responsive release of insulin at glucose concentration of 0, 1.0 and 3.0 mg mL−1, respectively. The nanoparticles from BP-1 as an example was depicted in Fig. 4B, and their sizes were maintained about 150 nm in absence of glucose. Within 70 h incubation at glucose concentration of 1.0 mg mL−1, the size of the nanoparticles was slightly expanded from 150 to 190 nm.
Comparatively, when the nanoparticles were incubated at 3.0 mg mL−1 glucose, the size was dramatically increased to 300 nm. These results were in consistent with the above discussed release profiles of insulin from the nanoparticles. One possible reason accounting for the size of these nanocarriers gradually expanding under the stimuli of 3.0 mg mL−1 glucose is supposed that the glucose-responsive phenylborate ester groups of the side chains detached from the polymer structure when they reacted with glucose molecules penetrating into the hydrophobic core of the nanoparticles, which led to the exposure of many hydroxyl groups to trigger the nanoparticles' swelling.
To explore their accurate glucose-sensitivity of these nanocarriers under normoglycemia and hyperglycemia, the release profile of insulin from MPEG-b-P(CL-g-PPBDMMA5)25 (BP-1) was carried out by altering the glucose concentration from 1.0 to 3.0 mg mL−1. As shown in Fig. 5A, insulin release percentage was controlled below 30% at 1.0 mg mL−1 for 70 h. Whereas, when the nanoparticles were at first incubated at 1.0 mg mL−1 glucose for 45 h and insulin release was close to 20%, adjusting glucose concentration to 3.0 mg mL−1 was conducted via adding glucose into the incubation vial. As a result, there was a rapid increase of insulin release, and the final release trend and amount were similar to that incubated at 3.0 mg mL−1 glucose at all time.
Pulsed release properties of the brush polymers in response to glucose are further investigated for in vivo application on treatment of hyperglycemia. Insulin alternative release experiment of BP-1 was conducted by simulating glucose concentration consecutive switch between 1.0 and 3.0 mg mL−1 every 8 h. As shown in Fig. 5B, due to the presence of retard period, no obvious disparity of insulin release was detected in the first two altering cycles of glucose concentration for 30 h. Subsequently, the nanoparticles incubated in the third pulsive cycle started to exhibit low release of insulin at 1.0 mg mL−1 glucose solution and the rapid release at 3.0 mg mL−1 glucose triggering. After the nanoparticles were placed back to 1.0 mg mL−1 glucose solution, low insulin release appeared again. Such pulsed insulin release was reproducibly obtained via the alternate incubation in 1.0 and 3.0 mg mL−1 glucose, and the cumulative release of insulin was close to 100%. Comparing to linear polymers, highly dense phenylborate ester groups due to such brush architecture might provide stronger hydrophobic interaction and form relatively stable nanoparticles with one compact hydrophobic core, which is not easy for glucose to penetrate. Although these nanoparticles can partially react with glucose molecules at 1.0 mg mL−1, most of glucose-responsive groups still maintain the hydrophobic interaction well, avoiding the immediate collapse of the nanoparticles. Only higher glucose concentration such as 3.0 mg mL−1 is powerful to break the nanoparticles' structure and result in complete release of insulin. Therefore, This brush architecture endows these nanoparticles from MPEG-b-P(CL-g-PPBDMMA) with prominent glucose-responsiveness difference under hyperglycemic and normoglycemic conditions at physiological pH. This intelligent insulin delivery system may have potential for the treatment of diabetes in response to the fluctuation of blood glucose concentration.
Stability of the released insulin and cell toxicity assays of the brush polymers
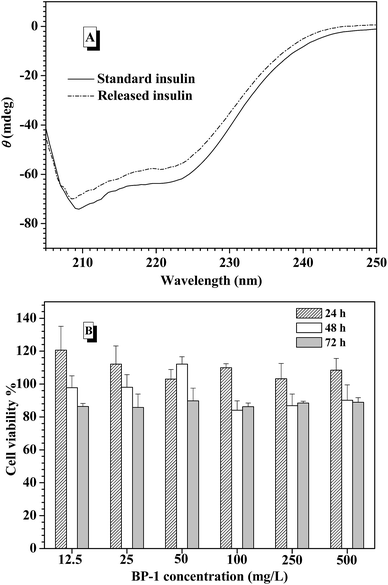
As reported,65 CD spectroscopy is an efficient technique to evaluate the conformational changes in insulin. Based on the principle that the ratio of the band at 208 nm arising from α-helix structure to that at 223 nm from β-structure ([Φ]208/[Φ]223) can qualitatively measure the overall conformational structure of insulin, the standard insulin and the released insulin suspended in the aqueous solution were analyzed using far UV-CD spectropolarimeter, respectively. As indicated in Fig. 6A, no significant conformational change was detected for the insulin released from the nanoparticles at pH 7.4 in comparison with the standard insulin, and [Φ]208/[Φ]223 for standard insulin and released insulin was 1.11 and 1.08, respectively. Furthermore, the spectral characteristics indicate that tertiary structure of released insulin was well preserved.To evaluate the potential toxicity of these copolymers, in vitro cytotoxicity assays of the nanoparticles from the brush polymers were performed using familiar NIH 3T3 mouse broblast cells and analyzed by MTS assay. Fig. 6B showed that the cells were exposed to various concentrations of the nanoparticles solutions from BP-1 and incubated for 24, 48 and 72 h, and MTS assay results of BP-2 and BP-3 were depicted in Fig. S12.† For all of the culture, the relative cell proliferation rates were close to 100% for 24 h, irrespective of copolymer nanoparticles concentration. The NIH 3T3 cell viability was still maintained more than 80% after 48 and 72 h cultivation as the nanoparticle concentration increased, indicating that the brush structure did not negatively impact cell proliferation. The cytotoxicity of the PCL-based brush polymers in the present work was compared with the polyacrylate-based polymers in the previous work by using L929 mouse fibroblast cells under the same condition.51 The cell viability of PCL-based brush polymers was always higher than that of polyacrylate-based polymer at different concentrations, suggesting that the introduction of PCL into the backbone successfully enhanced biocompatibility of the nanoparticles.
Degradation studies of the brush polymers MPEG-b-P(CL-g-PPBDMMA)
In our previous work, the nanoparticles were prepared by self-assembly of polyacrylate-based nonbiodegradable polymers, which were unfavorable for the metabolism of drug carriers.42,51 In this study, PCL-based brush polymers were used to prepare glucose-responsive nanoparticles for insulin delivery. Ester bonds in the backbone provided good biodegradability for the polymers, which was beneficial for the clearance of drug carriers in the form of degraded small molecules. The degradability of the nanoparticles from the brush polymers was at first investigated by monitoring release behavior of the encapsulated FITC–insulin in the presence of Novozym 435 lipase. Fig. 7 clearly showed the lipase-dependence release from the nanoparticles. For the nanoparticles from BP-1, no obvious insulin release trend was detected during 50 h incubation at 37 °C without Novozym 435. Whereas, nearly 100 percentage of the encapsulated insulin was rapidly released from the nanoparticles in identically measured time in the presence of the immobilized lipase. This indicates that the nanoparticles' disintegration caused by enzymatic degradation triggered insulin release. | ||
| Fig. 7 Insulin release profiles from BP-1 without and with lipase (0.1 mg mL−1) at pH 7.4 and 37 °C. | ||
To clarify the biodegradation process, the degradation products of BP-1 were verified by GPC analysis (Fig. 8A). Relative to the monomodal GPC curve of BP-1 with retention time of 9.4 min, GPC curves obtained from the incubated aliquots of BP-1 at physiological pH (7.4) at 37 °C in the presence of lipase would show multimode GPC curves with longer retention time. For examples, a major peak at 10.17 min and two minor peaks at 11.33 and 12.00 min were observed after 15 h incubation. The backbone partially degraded at this stage, and this major peak essentially corresponded to the hydrophilic block PEG species. According to species with elution time longer than that of PEG block, the two minor peaks were tentatively assigned to the repeating unit on the backbone (12.00 min, Mn = 913) and oligomers of side chain (11.33 min). After 30 h of incubation, significant occurrence of backbone degradation was indicated by the signal of BP-1 at 9.40 min completely vanishing and PEG residues remaining.
The degraded samples were taken out at predetermined time intervals and dialyzed against water (MWCO 3500) for 48 h. After lyophilization, the chemical component in the dialysis bag was determined by 1H NMR. Fig. 8B displayed different chemical structures of the dialyzed polymers responded to enzymatic degradation for determined time. Comparing to the chemical structure of the brush polymers without lipase (marked as 0 h in Fig. 8B), when the nanoparticles were incubated in lipase solution for 10 h, it was obviously weakened the signals between 7.25 and 7.67 ppm and that around 3.85 ppm were associated with phenyl ring signals from PBDMMA moiety and the methylene groups (e, f) bonding with borate ester, respectively. As degradation time extending, the proton signals of the hydrophobic segments were gradually diminished. After 30 h incubation, only signal from the hydrophilic PEG was detected in the dialysis bag. Combined with the all above experimental results, these nanocarriers formed from the brush polymers are biodegradable and have possibility to dissipate the concern of long-term toxicity in the body.
Conclusions
In summary, a class of novel degradable brush polymers with PCL as backbone and PPBDMMA as side chains was successfully prepared by grafting-from method. The molecular weights and PDI values of the macroinitiator MPEG–PBrCL and brush polymers MPEG-b-P(CL-g-PPBDMMA) were well controlled by ROP and ATRP, respectively. These brush polymers exhibited nanoscopic size in water. The nanocarriers from these brush polymers showed notable glucose concentration sensitivity, which was one rapid release of insulin from the nanocarriers with 3.0 mg mL−1 glucose (hyperglycemia) under physiological pH 7.4, while was comparatively inert release with 1.0 mg mL−1 glucose (normoglycemia). The controllable release of insulin was further conducted under pulsed trigger of 1.0 mg mL−1 and 3.0 mg mL−1 glucose. With benign degradability and low cytotoxicity, it showed good biocompatibility without long-term side effects. Based on the promising in vitro results, in vivo studies are required to further verify the applicability of MPEG-b-P(CL-g-PPBDMMA) as self-regulated insulin delivery in the treatment of diabetes.Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC, Grant No. 21174013; 21374005), the new century of the Ministry of Education, and BUCT Fund for Disciplines Improvement Plan (2050205).Notes and references
- S. S. Sheiko, B. S. Sumerlin and K. Matyjaszewski, Prog. Polym. Sci., 2008, 33, 759 CrossRef CAS.
- M. Zhang and A. H. E. Müller, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3461 CrossRef CAS.
- A. Bhattacharya and B. N. Misra, Prog. Polym. Sci., 2004, 29, 767 CrossRef CAS.
- H. Lee, J. Pietrasik, S. S. Sheiko and K. Matyjaszewski, Prog. Polym. Sci., 2010, 35, 24 CrossRef CAS.
- S. S. Sheiko and M. Moeller, Chem. Rev., 2001, 101, 4099 CrossRef CAS PubMed.
- M. B. Runge and N. B. Bowden, J. Am. Chem. Soc., 2007, 129, 10551 CrossRef CAS PubMed.
- Y. Xia, B. D. Olsen, J. A. Kornfield and R. H. Grubbs, J. Am. Chem. Soc., 2009, 131, 18525 CrossRef CAS PubMed.
- S. Panyuko, E. B. Zhulina, S. S. Sheiko, G. C. Randall and J. Brock, J. Phys. Chem. B, 2009, 113, 3750 CrossRef.
- K. Huang and J. Rzayew, J. Am. Chem. Soc., 2009, 131, 6880 CrossRef CAS PubMed.
- R. Djalali, S.-Y. Li and M. Schmidt, Macromolecules, 2002, 35, 4282 CrossRef CAS.
- J.-Z. Du, L.-Y. Tang, W.-J. Song, Y. Shi and J. Wang, Biomacromolecules, 2009, 10, 2169 CrossRef CAS PubMed.
- C. Cai, W. Zhu, T. Chen, J. Lin and X. Tian, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5967 CrossRef CAS.
- Y. Yu, C.-K. Chen, W.-C. Law, J. Mok, J. Zou, P. N. Prasad and C. Cheng, Mol. Pharm., 2013, 10, 867 CrossRef CAS PubMed.
- J. T. Zhang, Z. Cai, D. H. Kwak, X. Liu and S. A. Asher, Anal. Chem., 2014, 86, 9036 CrossRef CAS PubMed.
- J. Jung, J. C. Kim, Y. Rho, M. Kim, W. Kwon, H. Kim and M. Ree, ACS Appl. Mater. Interfaces, 2011, 3, 2655 CAS.
- J. Iruthayaraj, G. Olanya and P. M. Claesson, J. Phys. Chem. C, 2008, 112, 15028 CAS.
- X. Lu, T. H. Tran, F. Jia, X. Tan, S. Davis, S. Krishnan, M. M. Amiji and K. Zhang, J. Am. Chem. Soc., 2015, 137, 12466 CrossRef CAS PubMed.
- C. Hoertz, A. Birke, L. Kaps, S. Decker, E. Waechtersbach, K. Fischer, D. Schuppan, M. Barz and M. Schmidt, Macromolecules, 2015, 48, 2074 CrossRef CAS.
- Y. Yu, C. K. Chen, W. C. Law, H. Sun, P. N. Prasad and C. Cheng, Polym. Chem., 2015, 6, 953 RSC.
- J. Shi, J. G. Schellinger, R. N. Johnson, J. L. Choi, B. Chou, E. L. Anghel and S. H. Pun, Biomacromolecules, 2013, 14, 1961 CrossRef CAS PubMed.
- A. C. Engler, J. M. W. Chan, K. Fukushima, D. J. Coady, Y. Y. Yang and J. L. Hedrick, ACS Macro Lett., 2013, 2, 332 CrossRef CAS.
- American Diabetes Association, Diabetes Care, 2012, 35, S11 CrossRef PubMed.
- D. R. Owens, B. Zinman and G. B. Bolli, Lancet, 2001, 358, 739 CrossRef CAS.
- R. Ma and L. Shi, Polym. Chem., 2014, 5, 1503 RSC.
- W. Wu and S. Zhou, Macromol. Biosci., 2013, 11, 1464 CrossRef PubMed.
- R. Mo, T. Jiang, J. Di, W. Tai and Z. Gu, Chem. Soc. Rev., 2014, 43, 3595 RSC.
- I. Otsuka, T. Hongo, H. Nakade, A. Narumi, R. Sakai, T. Satoh, H. Kaga and T. Kakuchi, Macromolecules, 2007, 40, 8930 CrossRef CAS.
- W. Qi, X. Yan, J. Fei, A. Wang, Y. Cui and J. Li, Biomaterials, 2009, 30, 2799 CrossRef CAS PubMed.
- R. J. Ma, H. Yang, Z. Li, G. Liu, X. C. Sun, X. J. Liu, Y. L. An and L. Q. Shi, Biomacromolecules, 2012, 13, 3409 CrossRef CAS PubMed.
- W. Yang, X. Gao and B. Wang, Med. Res. Rev., 2003, 23, 346 CrossRef CAS PubMed.
- M. M. Zhou, J. D. Xie, S. T. Yan, X. M. Jiang, T. Ye and W. T. Wu, Macromolecules, 2014, 47, 6055 CrossRef CAS.
- D. Roy and B. S. Sumerlin, ACS Macro Lett., 2012, 1, 529 CrossRef CAS.
- A. Matsumoto, S. Ikeda, A. Karada and K. Kataoka, Biomacromolecules, 2003, 4, 1410 CrossRef CAS PubMed.
- A. Matsumoto, R. Yoshida and K. Kataoka, Biomacromolecules, 2004, 5, 1038 CrossRef CAS PubMed.
- A. Matsumoto, K. Yamamoto, R. Yoshida, K. Kataoka, T. Aoyagi and Y. Miyahara, Chem. Commun., 2010, 46, 2203 RSC.
- A. Matsumoto, T. Ishii, J. Nishida, H. Matsumoto, K. Kataoka and Y. Miyahara, Angew. Chem., Int. Ed., 2012, 51, 2124 CrossRef CAS PubMed.
- C. Cheng, X. G. Zhang, J. X. Xiang, Y. X. Wang, C. Zheng, Z. T. Lu and C. X. Li, Soft Matter, 2012, 8, 765 RSC.
- C. Cheng, X. Zhang, Y. Wang, L. Sun and C. Li, New J. Chem., 2012, 36, 1413 RSC.
- Y. Kotsuchibashi, R. V. C. Agustin, J. Y. Lu, D. G. Hall and R. Narain, ACS Macro Lett., 2013, 2, 260 CrossRef CAS.
- Q. Guo, Z. Wu, X. Zhang, L. Sun and C. Li, Soft Matter, 2014, 10, 911 RSC.
- Y. Yao, X. M. Wang, T. W. Tan and J. Yang, Soft Matter, 2011, 7, 7948 RSC.
- Y. Yao, L. Y. Zhao, J. J. Yang and J. Yang, Biomacromolecules, 2012, 13, 1837 CrossRef CAS PubMed.
- L. Y. Zhao, X. Zhang, Y. Yao, C. Yu and J. Yang, Macromol. Chem. Phys., 2014, 215, 1609 CrossRef CAS.
- T. Hoare and R. Pelton, Macromolecules, 2007, 40, 670 CrossRef CAS.
- B. L. Wang, R. J. Ma, G. Liu, Y. Li, X. J. Liu, Y. L. An and L. Q. Shi, Langmuir, 2009, 25, 12522 CrossRef CAS PubMed.
- B. L. Wang, R. J. Ma, G. Liu, Y. Li, X. J. Liu, Y. H. Gao, J. Y. Shen, Y. L. An and L. Q. Shi, Macromol. Rapid Commun., 2010, 31, 1628 CrossRef CAS PubMed.
- R. J. Ma, H. Yang, Z. Li, G. Liu, X. C. Sun, X. J. Liu, Y. L. An and L. Q. Shi, Biomacromolecules, 2012, 13, 3409 CrossRef CAS PubMed.
- H. Yang, X. Sun, G. Liu, R. Ma, Z. Li, Y. An and L. Shi, Soft Matter, 2013, 9, 8589 RSC.
- L. Sun, X. Zhang, C. Zheng, Z. Wu, X. Xia and C. Li, RSC Adv., 2012, 2, 9904 RSC.
- A. Matsumoto, K. Yamamoto, R. Yoshida, K. Kataoka, T. Aoyagi and Y. Miyahara, Chem. Commun., 2010, 46, 2203 RSC.
- G. Zhang, X. Zhuang, H. Shen, J. J. Yang and J. Yang, RSC Adv., 2014, 4, 49964 RSC.
- L. Zhao, J. X. Ding, C. S. Xiao, P. He, Z. H. Tang, X. Pang, X. L. Zhuang and X. S. Chen, J. Mater. Chem., 2012, 22, 12319 RSC.
- G. Liu, R. J. Ma, J. Ren, Z. Li, H. X. Zhang, Z. K. Zhang, Y. L. An and L. Q. Shi, Soft Matter, 2013, 9, 1636 RSC.
- R. Ma, X. Sun, X. Liu, Y. An and L. Shi, Aust. J. Chem., 2014, 67, 127 CrossRef CAS.
- H. Yang, R. Ma, J. Yue, C. Li, Y. Liu, Y. An and L. Shi, Polym. Chem., 2015, 6, 3837 RSC.
- H. Yang, C. Zhang, C. Li, Y. Liu, Y. An, R. Ma and L. Shi, Biomacromolecules, 2015, 16, 1372 CrossRef CAS PubMed.
- L. S. Nair and C. T. Laurencin, Prog. Polym. Sci., 2007, 32, 762 CrossRef CAS.
- S. Samarajeewa, R. Shrestha, Y. Li and K. L. Wooley, J. Am. Chem. Soc., 2012, 134, 1235 CrossRef CAS PubMed.
- J. Zou, C. C. Hew, E. Themistou, Y. Li, C. K. Chen, P. Alexandridis and C. Chen, Adv. Mater., 2011, 23, 4274 CrossRef CAS PubMed.
- S. Chen, X. Z. Zhang, S. X. Cheng, R. X. Zhuo and Z. W. Gu, Biomacromolecules, 2008, 9, 2578 CrossRef CAS PubMed.
- W. Wang, J. X. Ding, C. S. Xiao, Z. H. Tang, D. Li, J. Chen, X. L. Zhuang and X. S. Chen, Biomacromolecules, 2011, 12, 2466 CrossRef CAS PubMed.
- M. G. Li, W. Lu, L. J. Wang, C. X. Zhang, X. Q. Wang, A. P. Zheng and Q. Zhang, Int. J. Pharm., 2007, 329, 182 CrossRef CAS PubMed.
- I. Astafieva, X. F. Zhong and A. Eisenberg, Macromolecules, 1993, 26, 7339 CrossRef CAS.
- D. Roy, J. N. Cambre and B. S. Sumerlin, Chem. Commun., 2008, 2477 RSC.
- S. Lee, K. Kim, T. S. Kumar, J. Lee, S. K. Kim, D. Y. Lee, Y. K. Lee and Y. Byunm, Bioconjugate Chem., 2005, 16, 615 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01495j |
| This journal is © The Royal Society of Chemistry 2016 |