Synthesis of wrinkled graphene hybrids for enhanced visible-light photocatalytic activities†
Hua Xu*ab,
Jian Xin Xianga,
Pin Wua,
Yi Fei Lua,
Shuai Zhangac,
Zhuo Ying Xiea and
Zhong Ze Gu*ab
aState Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Si Pai Lou 2, Nanjing 210096, China. E-mail: huaxu@seu.edu.cn; gu@seu.edu.cn
bJiangSu Industrial Technology Research Institute, Nanjing 210096, China
cCenter for Low-dimensional Materials, Micro-nano Devices and System, Changzhou University, Changzhou 213164, China
First published on 29th April 2016
Abstract
Wrinkled graphene hybrids covalently modified with porphyrin were controllably synthesized and confirmed using Fourier transform infrared spectroscopy, UV-vis spectroscopy, fluorescence emission spectroscopy, thermogravimetric analysis, Raman spectroscopy, X-ray photoelectron spectroscopy, and transmission electron microscopy. Compared with the planar graphene hybrids covalently modified with porphyrin, the wrinkled graphene hybrids exhibit enhanced photocatalytic activity in the degradation of methylene blue under visible light. The cause of the formation of wrinkled graphene hybrids was analyzed and ascribed to the porphyrin interaction on the basal planes of graphene. This investigation might not only provide a new pathway toward the controllable synthesis of graphene hybrid materials with wrinkled morphology, but also a new approach to improving the photocatalytic performance of organic dye-sensitized graphene hybrid materials.
Introduction
Over the past decade, photocatalysis has attracted increasing attention because of its potential application in utilizing solar energy in solving energy depletion and global environmental issues. To date, inorganic semiconductors, such as TiO2, have been considered the most attractive photocatalysts because of their low cost, nontoxicity, and chemical stability. However, rapid recombination of photogenerated electron–hole pairs and the inability to utilize visible light limits their photocatalytic efficiency and practical applications. As a result, the development of novel visible-light-driven photocatalysts with high photocatalytic performance is currently a hot research topic.1Graphene, a two-dimensional material composed of sp2-hybridized carbon atoms packed in a honeycomb lattice, has recently been regarded as an ideal high-performance candidate for a photocatalyst carrier or promoter because of its superior properties, such as the fast room-temperature mobility of charge carriers, exceptional conductivity, and a large specific surface area, amongst others.2–7 Some graphene-based photocatalysts prepared by the modification of graphene with inorganic semiconductors8–12 or organic photosensitizers13–16 have demonstrated enhanced charge separation in charge transport and photocatalytic activity. The role of graphene in such photocatalyst systems is as an electron acceptor and transporter; it effectively suppresses the charge recombination and promotes charge transfer within these photocatalyst systems. Among these photocatalysts, the chemical functionalization of graphene with organic photosensitizers offers a practical strategy to combine the unique properties of each component and the potential to control the conjugate photoelectric properties such as light absorption, efficiency of charge transport and charge separation, and photocatalytic performance.17,18
Porphyrins are a class of large π-conjugated organic molecules with remarkably high extinction coefficients in the visible-light region, and they have potential photochemical electron-transfer ability. They have been widely used as highly efficient photosensitizers for photocatalysts.19,20 Various graphene/porphyrin nanocomposites have been prepared and used as highly efficient visible-light-driven photocatalysts. These include porphyrin/reduced graphene oxide (rGO) free-standing films,21 porphyrin noncovalently modified graphene oxide (GO) hybrids,22 graphene hybrids covalently modified with porphyrin23 or phthalocyanine,13,24 and graphene quantum dot/porphyrin nanocomposites.25 Although in comparison with each moiety of the hybrid, these graphene/porphyrin nanocomposites have demonstrated enhanced visible-light photocatalytic activity, their photocatalytic activity is still low, and further improvement of their photocatalytic performance remains a challenge. Combination with a noble metal has been used to enhance the photocatalytic activity of these graphene/porphyrin nanocomposites.26,27
Recently, crumpled rGO and curled graphene hybrid materials have demonstrated enhanced photoelectrochemical performance compared with their flat counterparts due to the high accessible surface area and rapid electron transfer via their open structure.28,29 These results prompted us to tune the morphology of graphene hybrid materials covalently modified with porphyrin to achieve improved photogenerated charge transport and separation, and photocatalytic activity. In the present work, a novel graphene hybrid covalently modified with porphyrin in a perpendicular face-to-edge alignment on the basal planes of graphene was prepared. The graphene hybrid materials can spontaneously form wrinkled morphology due to the porphyrin interaction on the basal planes of graphene. The visible-light photocatalytic activities of two graphene hybrid materials with planar and wrinkled morphology were investigated to illustrate the effect of the morphology on their photocatalytic activity.
Results and discussion
Synthesis of wrinkled graphene hybrids covalently modified with porphyrin
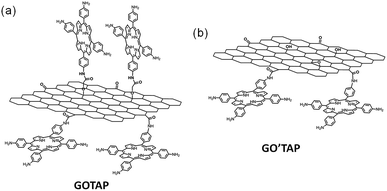
The curled graphene hybrid materials were prepared by covalent modification of trans-dihydroxotin(IV) porphyrin (SnP) in a parallel face-to-face alignment on the basal planes of graphene.29 The bonded SnP on the basal planes of graphene self-assembles easily via π–π, hydrophobic, and hydrogen-bond interactions. Such interactions induce the SnP bonded on the basal planes of graphene to close to each other, in an edge-to-edge way, which is also referred to as the J-aggregation of porphyrin,30,31 and thus strain the graphene sheet, leading to a curled graphene hybrid material. Here, to control the self-assembly of porphyrin bonded on the basal planes of graphene in a face-to-face way, which is also referred to as H-aggregation of porphyrin,30,31 tetra(4-aminophenyl)porphyrin (TAP) was used to covalently bond to the basal planes of graphene in a perpendicular edge-to-face alignment as well as to the edge in an edge-to-edge alignment (Fig. 1).GO was first treated with chloroacetic acid under strongly basic conditions to activate epoxide and hydroxyl groups, and introduce carboxyl groups on the basal planes of GO.32 Carboxylic-functionalized GO (GOCOOH), activated upon treatment with thionyl chloride, was available to react with TAP by formation of amide bonds to achieve perpendicular edge-to-face alignment of porphyrin on the basal planes of graphene and edge-to-edge alignment of porphyrin at the edges (GOTAP) (Fig. 1). For comparison, the graphene hybrid material (GO′TAP) with only an edge-to-edge alignment of porphyrin at the edges was prepared by the reaction of the activated GO with TAP.
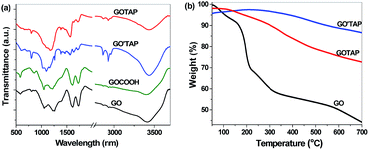
The forms of the two graphene/porphyrin hybrids were characterized by Fourier transform infrared (FTIR) spectroscopy. In the FTIR spectra (Fig. 2(a)), GO and GOCOOH have characteristic absorption bands corresponding to the O–H stretching vibration at 3410 cm−1, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the carbonyl and carboxylic groups at 1730 cm−1, the C–OH stretching vibration at 1380 cm−1, and the C–O stretching vibration at 1049 cm−1. After covalent functionalization with porphyrins, two new peaks appeared at 1567 cm−1 and 1717 cm−1, corresponding to the C
O stretching vibration of the carbonyl and carboxylic groups at 1730 cm−1, the C–OH stretching vibration at 1380 cm−1, and the C–O stretching vibration at 1049 cm−1. After covalent functionalization with porphyrins, two new peaks appeared at 1567 cm−1 and 1717 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations of porphyrins, respectively, and the peak of the C–O stretching vibration at 1049 cm−1 shifts to 1108 cm−1. The peak of the C–OH stretching vibration at 1380 cm−1 disappears and a peak at 1640 cm−1 appears because of the formation of amide linkages.33 These changes in the FTIR spectra indicate that TAP was covalently bonded to GO and GOCOOH, and GO′TAP and GOTAP hybrids were formed.
N vibrations of porphyrins, respectively, and the peak of the C–O stretching vibration at 1049 cm−1 shifts to 1108 cm−1. The peak of the C–OH stretching vibration at 1380 cm−1 disappears and a peak at 1640 cm−1 appears because of the formation of amide linkages.33 These changes in the FTIR spectra indicate that TAP was covalently bonded to GO and GOCOOH, and GO′TAP and GOTAP hybrids were formed.
XPS spectra of GO′TAP and GOTAP, compared with that of GO, show a new peak at around 399 eV that is ascribed to N 1s (Fig. S1(a)–(c)†).34 N is a component element of tetra(4-aminophenyl)porphyrin, but not of GO, so it can be concluded that the porphyrin have bonded on the graphene sheet of GOTAP and GO′TAP. Detailed analysis of the XPS spectra provides essential and useful information for the covalent attachment of porphyrin moieties on the graphene. The C 1s spectrum of GO showed that the lower binding energy at around 284.4 eV belongs to C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C carbons, and the higher binding energy at around 286.3 eV corresponds to C–OH from epoxide and hydroxyl groups (Fig. S1(d)†). Moreover, the peaks at around 286.8 and 289.1 eV are ascribed to the C
C carbons, and the higher binding energy at around 286.3 eV corresponds to C–OH from epoxide and hydroxyl groups (Fig. S1(d)†). Moreover, the peaks at around 286.8 and 289.1 eV are ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O arising from the carboxyl group. After modification with porphyrin, two new peaks at around 285.3 and 288.3 eV appeared in the C 1s spectra of GO′TAP and GOTAP (Fig. S1(e) and (f)†), which were attributed to C–N and C
O arising from the carboxyl group. After modification with porphyrin, two new peaks at around 285.3 and 288.3 eV appeared in the C 1s spectra of GO′TAP and GOTAP (Fig. S1(e) and (f)†), which were attributed to C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N units, respectively.34 Moreover, the presence of a peak at around 287.2 eV, corresponding to the N–C
N units, respectively.34 Moreover, the presence of a peak at around 287.2 eV, corresponding to the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit, indicated that TAP was covalently bonded to GO and GOCOOH by an amide linkage.13 The N 1s XPS spectra of GOTAP and GO′TAP can be decomposed into three components at around 398.1 eV, 399.3 eV, and 400.7 eV (Fig. S1(g) and (h)†), which correspond to the N atoms in the C–N, N–H, and C
O unit, indicated that TAP was covalently bonded to GO and GOCOOH by an amide linkage.13 The N 1s XPS spectra of GOTAP and GO′TAP can be decomposed into three components at around 398.1 eV, 399.3 eV, and 400.7 eV (Fig. S1(g) and (h)†), which correspond to the N atoms in the C–N, N–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N units, respectively. These changes in the XPS spectra also indicated that TAP was covalently bonded to GO and GOCOOH, and GO′TAP and GOTAP hybrids were formed.
N units, respectively. These changes in the XPS spectra also indicated that TAP was covalently bonded to GO and GOCOOH, and GO′TAP and GOTAP hybrids were formed.
The amount of porphyrin attached to the GO′TAP and GOTAP nanohybrids was determined by thermogravimetric analysis (TGA) (Fig. 2(b)). The TGA curves of GO show a slight mass decrease from room temperature to 150 °C because of the evaporation of absorbed water and a significant decrease from 150 °C to 350 °C because of the decomposition of labile oxygen functional groups, such as hydroxyl, epoxy, and carbonyl groups, and the removal of the more stable oxygen functionalities. After modification with TAP, the resultant GO′TAP and GOTAP are thermally more stable than GO. When heated from 250 °C to 550 °C, they lose approximately 8% and 15% weight, respectively. This weight loss corresponds to the loss of TAP molecules covalently attached to graphene.35 The greater amount of attached porphyrin in GOTAP is consistent with the porphyrin of GOTAP being attached not only on the edges, but also on the basal planes of graphene.
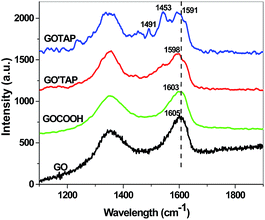
The Raman spectra of GO and GOCOOH all display characteristic D and G bands (Fig. 3), with an ID/IG ratio of 0.80 and 0.95, respectively. Unlike GO and GOCOOH, the Raman spectra of the GO′TAP and GOTAP materials exhibited an enhancement of the D peak, and the ID/IG ratio increased to 1.02 and 1.0, respectively, indicating successful covalent attachment of TAP onto graphene.29,33 Furthermore, two new bands at 1491 cm−1 and 1543 cm−1 appear in the Raman spectra of the GO′TAP and GOTAP nanohybrids. They are assigned to the pyrrole ring Cb–Cb (NH) vibration and pyrrolidine ring Cb–Cb (N) vibration in TAP, respectively.36 The intensities of those two peaks in the Raman spectra of GOTAP are much stronger than in the case of GO′TAP. This result indicates that there are more attached porphyrin molecules in the case of GOTAP than in GO′TAP. This is in agreement with the TGA results.
The G peak position of the GO′TAP and GOTAP nanohybrids exhibited a shift to lower frequencies (ca. 7 cm−1 and 12 cm−1, respectively), compared with GO and GOCOOH. In general, the G band of graphene in the Raman spectrum is known to be shifted to lower frequencies (softening) when hybridized with an electron-donor component or to higher frequencies (stiffening) when hybridized with an electron-acceptor component.21 This shift to a lower frequency in our GO′TAP and GOTAP hybrids confirms the occurrence of charge transfer between TAP and graphene in the two hybrid materials, where porphyrin acts as an electron donor and graphene as an electron acceptor. The larger shift to a lower frequency in GOTAP also indicates that there is stronger charge transfer here between the porphyrins and graphene, which is very important for high photocatalytic activity.
Optical properties of GO′TAP and GOTAP
The UV-vis absorption spectra of GO′TAP and GOTAP both have a broad signal, which monotonically decreases from the UV to the visible region (Fig. 4). This is attributed to graphene, and a characteristic Soret band corresponding to the covalently linked porphyrin units. The broadened visible-light absorption in the nanohybrids could possibly provide more photocharges required for the photocatalytic reactions. Compared with TAP and a control sample, which is a mixture of GOCOOH and TAP according to the proportion of the TGA results, the absorption spectra of GO′TAP and GOTAP both exhibit noticeable broadening and a blue shift of the Soret band absorption (from 433 nm to 428 nm and from 433 nm to 424 nm, respectively). These results further support the formation of a covalently linked graphene/porphyrin nanohybrid (GO′TAP and GOTAP). The larger blue shift of GOTAP also indicates strong interaction between the porphyrins, which is consistent with the wrinkled morphologies induced by porphyrin interaction as described in the following. Moreover, although GO′TAP has stronger graphene absorption in the UV region than GOTAP, the Soret band absorption of GOTAP is much stronger than that of GO′TAP, mainly caused by the covalently bonded TAP units. This indicates that in GOTAP there are more attached porphyrin molecules than in GO′TAP. This is in agreement with TGA and Raman spectra results.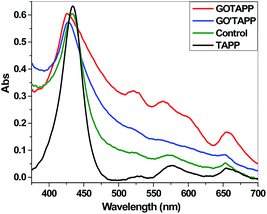 | ||
| Fig. 4 UV-vis absorption spectra; the control sample is a mixture of GOCOOH with TAP according to the proportion from the TGA results. | ||
The emission spectrum of TAP in DMF shows a strong fluorescence emission peak centered at 685 nm, because of the conjugated structure of TAP (Fig. 5). The fluorescence spectrum of the control sample, compared with that of TAP, exhibits 21% quenching of the fluorescence emission, while the GO′TAP and GOTAP hybrid materials exhibit almost complete quenching. The significant fluorescence quenching indicates that there is a strong interaction between the excited state of porphyrin and the graphene moieties in the nanohybrid. Efficient photoinduced electron transfers have been used to illustrate the significant fluorescence quenching of the excited porphyrin.37 Porphyrins represent a class of typical electron donors, while graphene is a favorable electron acceptor. Upon photoexcitation, the intramolecular donor–acceptor interaction between the two moieties of TAP and graphene in the nanohybrid may encounter charge transfer from the photoexcited singlet TAP to the graphene moiety, the result of which is the observed fluorescence quenching and energy release. The efficient charge transfer in the nanohybrid affords a high possibility of photogenerated carrier separation and holds promise for high photocatalytic activity.
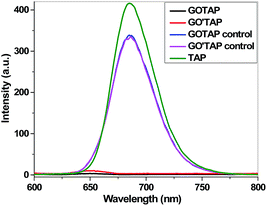 | ||
| Fig. 5 Fluorescence spectra of TAP, GO′TAP, GOTAP, and the control sample with the matching absorbances at the excitation wavelength. | ||
Wrinkled morphology of GOTAP and the mechanism of formation
Transmission electron microscopy (TEM) images of GO, GOCOOH, and GO′TAP all show regular, flat morphologies (Fig. 6(a)–(c)). However, the TEM images of GOTAP show nanoscale wrinkled morphologies (Fig. 6(d) and (e)). Magnified TEM images show that the wrinkled morphology of GOTAP mainly originates from the corrugation and folding of the graphene sheet (Fig. 6(f)). Chemical functional groups on the graphene sheet have been reported to induce the wrinkling of graphene.38 Upon comparing the chemical structure of GO′TAP with that of GOTAP (Fig. 1), we deduced that the wrinkled GOTAP may be attributed to the porphyrins on the basal planes of graphene. The porphyrins are very close and could easily self-assemble via π–π, hydrophobic, and hydrogen-bond interactions (Fig. 6(g)). This has been confirmed by UV-vis absorption spectra. Such interactions would induce a strain in the graphene sheet and lead to wrinkle formation in the graphene sheet.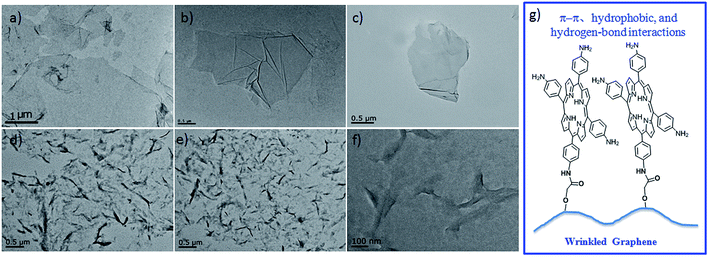 | ||
| Fig. 6 TEM images of (a) GO, (b) GOCOOH, (c) GO′TAP, and (d–f) GOTAP. (g) Porphyrin interactions of the wrinkled GOTAP. | ||
Recent studies have revealed that in the self-assembly of porphyrin, two main self-assembled aggregation modes are easily formed:30,31 one is H-aggregation, in which the porphyrins are arranged in a face-to-face manner; another is J-aggregation, in which the porphyrins are arranged in an edge-to-edge manner. It is now widely accepted that the H-aggregation of porphyrin gives rise to a blue-shifted extinction band with respect to the monomer porphyrin, while the J-aggregation of porphyrin causes the appearance of a narrow red-shifted extinction band. In our previous work,29 we reported that graphene hybrid materials covalently modified with porphyrin in a parallel face-to-face alignment on the basal planes of graphene (GOSnP) exhibited obvious curled morphology owing to porphyrin interactions. The absorption spectrum of GOSnP, compared with the spectrum of monomer porphyrin (SnP), exhibits noticeable broadening and a 7 nm red shift of the Soret band absorption. This result supported the idea that SnP bonded on the basal planes of graphene self-assembles in J-aggregation, in which the porphyrins are arranged in an edge-to-edge manner, and this self-assembly mode can induce the curl of the graphene sheet. Here, TAP was covalently bonded to the basal planes of graphene in a perpendicular edge-to-face alignment to prepare the graphene hybrid material (GOTAP). The absorption spectrum of the GOTAP, compared with the spectrum of TAP, exhibited noticeable broadening and a 9 nm blue shift of the Soret band absorption (Fig. 4). This result indicated that the TAP bonded on the basal planes of graphene self-assembles in H-aggregation, while the TAP bonded on the basal planes of graphene self-assembles in a face-to-face manner and this self-assembly mode induces the wrinkling of the graphene sheet (Fig. 6(g)). These results also indicated that the morphology of graphene hybrid materials can be controlled by the self-assembly mode of porphyrin bonded on the basal planes of graphene. This provides a simple and effective approach to large-scale tuning of the extrinsic morphology of graphene materials. Recent studies have revealed that crumpled, rippled, and curled graphene material can have notably improved electron mobility, chemical reactivity, and electrochemical properties.28,29,39,40 Therefore, it can be expected that wrinkled GOTAP will have improved photocatalytic performance.
Visible-light photodegradation of methylene blue
The photocatalytic performance of the as-prepared nanohybrids was evaluated in the degradation of methylene blue (MB) under visible-light irradiation at room temperature. The results are shown in Fig. 7(a). To compare the photocatalytic activity, all samples were prepared with the same concentrations. Under visible-light irradiation, without a photocatalyst, the absorption intensity of MB displayed a negligible decrease, which suggested that the self-sensitized photodegradation of MB could hardly occur when no photocatalyst was involved. A control experiment was also performed in the presence of the nanohybrids, without light irradiation, and no obvious MB degradation was observed. When GO, TAP, and the two graphene/porphyrin nanohybrids were used, a decrease in the absorption intensity of MB with irradiation time was observed under visible-light irradiation. Compared with GO and TAP, the two graphene/porphyrin nanohybrids exhibited a more significant decrease in the absorption intensity of MB under the same experimental condition, indicating that the resultant graphene/porphyrin nanohybrids have enhanced visible-light photocatalytic performance. This result is consistent with the study results reported previously.21,22 Moreover, the decrease in the absorption intensity of MB in the presence of GOTAP was much higher than in the case of GO′TAP under the same experimental conditions, which indicated that wrinkled GOTAP has better photocatalytic performance than GO′TAP.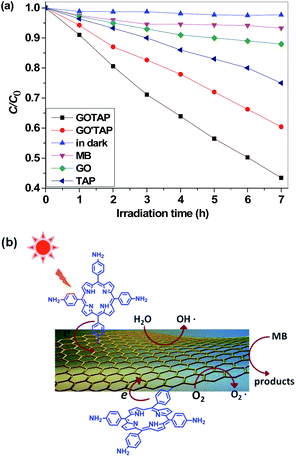 | ||
| Fig. 7 (a) Photocatalytic degradation of MB with different samples under visible-light irradiation (λ > 400 nm). (b) Photocatalytic mechanism for the GOTAP nanohybrid. | ||
The high photocatalytic performance of the graphene/porphyrin nanohybrids is attributed to the efficient charge separation in the nanohybrids.21 Compared with GO′TAP, the greater amount of porphyrin molecules was covalently linked in the GOTAP, which has been confirmed by the TGA and Raman spectra results. More attached porphyrin molecules in the GOTAP would produce more electron excitation and electron–hole pairs under visible-light irradiation. Because of the superior electron-accepting and electron-transporting properties of graphene, stronger charge transfer between the porphyrins and graphene moiety occurs in the GOTAP nanohybrid, which has been confirmed by the Raman and fluorescence spectra. Moreover, compared with planar GO′TAP, the wrinkled GOTAP could provide higher accessible surface area in solution for MB absorption.28 Thus, enhanced photocatalytic activity was achieved for the wrinkled graphene hybrids in the degradation of MB under visible light.
A possible reaction mechanism for the photodegradation of MB under visible-light irradiation over our graphene/porphyrin hybrids is illustrated in Fig. 7(b). Under visible-light irradiation, the porphyrin moiety is excited, producing electron excitation and electron–hole pairs. Because of the perfect conductivity of graphene and the interfacial equilibrium of energy levels, the transfer of electrons from porphyrin to graphene is theoretically favorable. As a combination of an electron acceptor and a transporter, graphene can efficiently inhibit the recombination of charge carriers. Therefore, the electrons transferred to graphene can be scavenged by the dissolved oxygen to form superoxygen radicals.21 Moreover, the holes in graphene may react with water (or hydroxyl ions) to form hydroxyl free radicals. The active species of hydroxyl and superoxide radicals can directly oxidize MB molecules to form CO2 and H2O.
Conclusions
In conclusion, we designed and prepared novel graphene nanohybrids covalently modified with porphyrin in a perpendicular face-to-edge alignment on the basal planes of graphene. The resultant graphene hybrid materials can spontaneously form a wrinkled morphology due to the strain induced by the porphyrin interaction on the basal planes of graphene. Compared with the planar graphene nanohybrids covalently modified with porphyrin at the edges of graphene, the wrinkled graphene nanohybrids exhibit enhanced charge transfer and photocatalytic activity, as is evident in the degradation of MB under visible light. This research provides a new approach to improving the photocatalytic performance of organic dye-sensitized graphene hybrid materials based on their extrinsic morphology and structure. It opens new possibilities to provide some insight into the design of high-performance graphene-based photocatalysts and other applications.Experimental
Materials and reagents
All solvents and chemicals were of reagent grade and used without further purification. Ultrapure water was obtained from a Millipore water purification system (≥18 MΩ, Milli-Q, Millipore). Graphene oxide (GO, purity 99%, single layer ratio 99%) were purchased from Nanjing XFNano Materials Technology Co., Ltd and used as received. Carboxyl-functionalized graphene oxide (GOCOOH)32 and 5,10,15,20-meso-tetra(4-aminophenyl)porphyrin (TAP)41,42 were prepared according to the literature.Synthesis of wrinkled graphene hybrids covalently modified with porphyrin
GOCOOH (30 mg) in SOCl2 (20 mL) was refluxed in the presence of DMF (0.5 mL) at 70 °C for 24 h under nitrogen atmosphere. At the end of the reaction, excess SOCl2 and solvent were removed by distillation and the product was washed with dry tetrahydrofuran. In the presence of triethylamine (Et3N, 0.5 mL), the above product was allowed to react with 5,10,15,20-meso-tetra(4-aminophenyl)porphyrin (TAP, 30 mg) in DMF (30 mL) at 130 °C for 72 h under nitrogen. After the reaction, the solution was cooled to room temperature. The product was isolated by filtration on a Nylon membrane (0.22 μm). The excess TAP and other impurities were removed through five washing cycles, which included sonication, filtration (discarding the filtrate), and re-suspension of the solid in DMF. The precipitate was washed with CHCl3 five times, following the above procedure. UV-vis spectroscopy was used to check the filtrate to ensure no TAP existed in the final washing. The graphene hybrid was then washed with small quantity of H2O to remove Et3N·HCl, and finally dried under vacuum.Visible-light photodegradation of methylene blue
The photodegradation of methylene blue (MB) dyes was observed based on the absorption spectroscopic technique. In a typical procedure, an aqueous solution of MB (10 ppm, 30 mL) and the photocatalyst (3 mg) was placed in a 50 mL cylindrical quartz vessel. Under ambient conditions, the suspension was continuously stirred for 12 h in the dark to reach an adsorption–desorption equilibrium between the MB and the photocatalyst. Then the photoreaction vessel was exposed to the visible-light irradiation produced by a 500 W halogen lamp with an UV-cutoff (L-42, Hoya Corp) and an IR-cutoff filter. The light intensity was 10 mW cm−2, which was monitored and corrected by the optical power meter. At a given time interval of irradiation, about 2 mL aliquots were taken out from the reaction system and then centrifuged to remove the photocatalysts completely. The concentration of MB in the aliquot was analyzed by using a UV-vis spectrophotometer at its characteristic wavelength (λ = 665 nm). The blank experiments without photocatalyst or without illumination were also carried out for comparison.Characterization and measurements
IR spectral measurements were recorded on a Nicolet 5700 FTIR spectrometer. All infrared (IR) samples were prepared as KBr disc using spectroscopic grade KBr. UV-vis absorbance spectroscopy was recorded on a U-4100 spectrometer (Hitachi, Japan). Steady-state fluorescence spectroscopy was measured on an F-7000 fluorescence spectrophotometer (Hitachi, Japan). TGA measurements were run on a SDT Q600 Simultaneous DSC-TGA Instrument under N2 purge with a heating rate of 10 °C min−1. Raman spectra were measured on a Renishaw Invia Raman Microscope with Ar+ radiation (excitation at 532 nm). Samples for Raman measurements were prepared by casting a few drops of the dispersions of the graphene materials on clean glass substrates and were then dried. The X-ray photoelectron spectroscopy (XPS) measurement was carried out using an PHI 5000 Versaprobe spectrometer fitted with a monochromatic Al Kα X-ray source (hν = 1486.6 eV). Peak fitting of XPS spectra was performed with the help the XPSpeak software using Shirley background. TEM images were obtained on a JEM-2100 instrument operating at 200 kV. TEM samples were prepared by drop casting the dispersions of the graphene materials on the carbon-coated Cu grid and allowing them to dry in air in the ambient condition.Acknowledgements
The research is primarily funded by NSFC (Grants 51573025) and Natural Science Foundation of Jiangsu Province (Grant No. BK20151454 and BK20150024).Notes and references
- M. D. Hernández-Alonso, F. Fresno, S. Suárez and J. M. Coronado, Energy Environ. Sci., 2009, 2, 1231–1257 Search PubMed
.
- N. Zhang, M. Q. Yang, S. Q. Liu, Y. G. Sun and Y. J. Xu, Chem. Rev., 2015, 115, 10307–10377 CrossRef CAS PubMed
.
- N. Zhang, Y. H. Zhang and Y. J. Xu, Nanoscale, 2012, 4, 5792–5813 RSC
.
- D. Chen, H. Zhang, Y. Liu and J. H. Li, Energy Environ. Sci., 2013, 6, 1362–1387 CAS
.
- X. Q. An and J. C. Yu, RSC Adv., 2011, 1, 1426–1434 RSC
.
- L. Han, P. Wang and S. J. Dong, Nanoscale, 2012, 4, 5814–5825 RSC
.
- G. C. Xie, K. Zhang, B. D. Guo, Q. Liu, L. Fang and J. R. Gong, Adv. Mater., 2013, 25, 3820–3839 CrossRef CAS PubMed
.
- Q. J. Xiang, J. G. Yu and M. Jaronie, Chem. Soc. Rev., 2011, 41, 785–796 Search PubMed
.
- Y. H. Ng, A. Iwase, N. J. Bell, A. Kudo and R. Amal, Catal. Today, 2011, 164, 353–357 CrossRef CAS
.
- Q. Li, X. Li, S. Wageh, A. A. Al-ghamdi and J. G. Yu, Adv. Energy Mater., 2015, 5, 1500010–1500027 Search PubMed
.
- X. T. Chang, L. H. Dong, Y. S. Yin and S. B. Sun, RSC Adv., 2013, 3, 15005–15013 RSC
.
- X. M. Tu, S. L. Luo, G. X. Chen and J. H. Li, Chem.–Eur. J., 2012, 18, 14359–14366 CrossRef CAS PubMed
.
- Y. Wang, A. D. Zhou, Y. Jiang, X. Y. Chen and J. B. He, RSC Adv., 2015, 5, 37823–37829 RSC
.
- M. S. Zhu, Y. P. Dong, B. Xiao, Y. K. Du, P. Yang and X. M. Wang, J. Mater. Chem., 2012, 22, 23773–23779 RSC
.
- M. Latorre-Snchez, C. Lavorato, M. Puche, V. Forns, R. Molinari and H. Garcia, Chem.–Eur. J., 2012, 18, 16774–16783 CrossRef PubMed
.
- B. Xiao, X. M. Wang, H. Huang, M. S. Zhu, P. Yang, Y. Wang and Y. K. Du, J. Phys. Chem. C, 2013, 117, 21303–21311 CAS
.
- T. Kuila, S. Bose, A. K. Mishra, P. Khanra, N. H. Kim and J. H. Lee, Prog. Mater. Sci., 2012, 57, 1061–1105 CrossRef CAS
.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156–6214 CrossRef CAS PubMed
.
- T. Shiragami, J. Matsumoto, H. Inoue and M. Yasuda, J. Photochem. Photobiol., C, 2005, 6, 227–248 CrossRef CAS
.
- M. Silva, M. E. Azenha, M. M. Pereira, H. D. Burrows, M. Sarakha, C. Forano, M. F. Ribeiro and A. Fernandes, Appl. Catal., B, 2010, 100, 1–9 CrossRef CAS
.
- Y. Z. Chen, Z. H. Huang, M. B. Yue and F. Y. Kang, Nanoscale, 2014, 6, 978–985 RSC
.
- P. P. Guo, P. L. Chen and M. H. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 5336–5345 CAS
.
- J. H. Sun, D. Meng, S. D. Jiang, G. F. Wu, S. K. Yan, J. X. Geng and Y. Huang, J. Mater. Chem., 2012, 22, 18879–18886 RSC
.
- D. D. Wang, J. Huang, X. Li, P. Yang, Y. K. Du, C. M. Goh and C. Lu, J. Mater. Chem. A, 2015, 3, 4195–4202 CAS
.
- Q. Lu, Y. J. Zhang and S. Q. Liu, J. Mater. Chem. A, 2015, 3, 8552–8558 CAS
.
- M. S. Zhu, Z. Li, B. Xiao, Y. T. Lu, Y. K. Du, P. Yang and X. M. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 1732–1740 CAS
.
- H. Y. Li, Y. W. Zhang, G. H. Chang, S. Liu, J. Q. Tian, Y. L. Luo, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, ChemPlusChem, 2012, 77, 545–550 CrossRef CAS
.
- J. Y. Luo, H. D. Jang, T. Sun, L. Xiao, Z. He, A. P. Katsoulidis, M. G. Kanstzidis, J. M. Gibson and J. X. Huang, ACS Nano, 2011, 5, 8943–8949 CrossRef CAS PubMed
.
- H. Xu, P. Wu, C. Liao, C. G. Lv and Z. Z. Gu, Chem. Commun., 2014, 50, 8951–8954 RSC
.
- M. Shirakawa, S. Kawano, N. Fujita, K. Sada and S. Shinkai, J. Org. Chem., 2003, 68, 5037–5044 CrossRef CAS PubMed
.
- G. F. Lu, X. M. Zhang, X. Cai and J. Z. Jiang, J. Mater. Chem., 2009, 19, 2417–2424 RSC
.
- X. M. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. J. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS PubMed
.
- A. J. Wang, L. L. Long, W. Zhao, Y. L. Song, M. G. Humphrey, M. P. Cifuentes, X. Z. Wu, Y. S. Fu and C. Zhang, Carbon, 2013, 53, 327–338 CrossRef CAS
.
- A. J. Wang, Y. Wang, Z. G. Xiao, Y. L. Song, M. P. Cifuentes, M. G. Humphrey and C. Zhang, Nano Res., 2015, 8, 870–886 CrossRef CAS
.
- N. Karousis, A. S. D. Sandanayaka, T. Hasobe, S. P. Economopoulos, E. Sarantopouloua and N. Tagmatarchis, J. Mater. Chem., 2011, 21, 109–117 RSC
.
- D. L. Akins, C. Guo and H. R. Zhu, J. Phys. Chem., 1993, 97, 3974–3977 CrossRef CAS
.
- Y. F. Xu, Z. B. Liu, X. L. Zhang, Y. Wang, J. G. Tian, Y. Huang, Y. F. Ma, X. Y. Zhang and Y. S. Chen, Adv. Mater., 2009, 21, 1275–1279 CrossRef CAS
.
- Q. B. Zheng, Y. Geng, S. J. Wang, Z. G. Li and J. K. Kim, Carbon, 2010, 48, 4315–4322 CrossRef CAS
.
- D. W. Boukhvalov and M. I. Katsnelson, J. Phys. Chem. C, 2009, 113, 14176–14178 CAS
.
- J. F. Zang, S. Ryu, N. Pugno, Q. M. Wang, Q. Tu, M. J. Buehler and X. H. Zhao, Nat. Mater., 2013, 12, 321–325 CrossRef CAS PubMed
.
- H. Xu and J. Y. Zheng, Macromol. Chem. Phys., 2010, 211, 2125–2131 CrossRef CAS
.
- H. Xu, K. D. Cao, H. B. Ding, Q. F. Zhong, H. C. Gu, Z. Y Xie, Y. J. Zhao and Z. Z. Gu, ACS Appl. Mater. Interfaces, 2012, 4, 6752–6757 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01458e |
| This journal is © The Royal Society of Chemistry 2016 |