In situ growth of silver nanowires on reduced graphene oxide sheets for transparent electrically conductive films
Zhonglin Luo,
Zengping Cai,
Yanbin Wang,
Yupeng Wang and
Biaobing Wang*
Sch. Mat. Sci. & Eng., Jiangsu Collaborat. Innovat. Ctr. Photovolat Sci. & Eng., Changzhou Univ, Changzhou 213164, Jiangsu, China. E-mail: biaobing@cczu.edu.cn; Fax: +86-519-86330075; Tel: +86-519-86330075
First published on 7th April 2016
Abstract
In situ growth of silver nanowires (AgNWs) on the surface of functionalized-graphene (rGO) nanosheets is achieved through a synchronous reduction of graphene oxide and silver ions using sodium citrate as the chemical reduction agent in the presence of poly(diallyldimethyl ammonium chloride). The prepared AgNW–rGO hybrids are characterized by zeta potential, X-ray diffraction, Raman spectroscopy, transmission and scanning electron microscopy, atomic force microscopy, respectively. The stable AgNW–rGO aqueous solution is readily used to fabricate highly transparent, flexible and conductive films. The AgNW–rGO/PVA films show an electrical conductivity of 19.5 S m−1 with 67.7% light transmittance at a wavelength of 550 nm.
1. Introduction
Recently, monolayer or few layer graphene has been a subject of great interest not only due to its interesting physical and chemical properties but also due to its potential application for future electronic and optoelectronic devices.1–5 Moreover, heterostructures consisting of carbon materials and metals or metal oxides have received considerable attention due to the potential influence of their fundamental properties on each other.6–9 Silver (Ag) is widely used because of its unique optical properties, surface plasmon resonance, and the highest electrical and thermal conductivity among metals. More recently, a novel architecture that consists of one-dimensional (1D) silver nanowires (AgNWs) and two-dimensional (2D) graphene sheets has attracted increasing concerns since long 1D AgNWs play the role of framework to enhance the electrical conductivity and mechanical flexibility of the hybrid films,10–15 the majority of which were performed by means of post-processing or ex situ process. Although there were some reports16–24 on the anchoring of silver nanoparticles into the reduced graphene oxide (rGO), there is few reports about the decorating directly the AgNWs on the surface of rGO sheets.25In this paper, we reported a facile one-pot method to obtain the decoration of AgNWs on the rGO sheets (AgNW–rGO) by synchronous reduction of graphene oxide and silver ions using sodium citrate as the reduction agent and poly(diallyldimethyl ammonium chloride) (PDDA) as electrostatic stabilizing agent. Water-soluble poly(vinyl alcohol) (PVA) was selected as the substrate for preparing transparent conductive films (TCFs) because PVA possesses outstanding transmittance and mechanical properties. This simple one-pot hydrothermal reduction process produced AgNW–graphene hybrid TCFs with high electrical conductivity and excellent optical transmittance.
2. Experiments
2.1 Chemicals and materials
Graphite was obtained from Alfa Aesar (Beijing, China). Poly(diallyldimethyl ammonium chloride) (PDDA) (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w = 40
w = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 35 wt% in water) was purchased from Aldrich (Shanghai, China). AgNO3 and sodium citrate trihydrate were obtained from Shanghai Chemical Reagent Co. (Shanghai, China). All of the reagents were used as received. Glassware used in the following procedures was cleaned in a bath of freshly prepared 3
000, 35 wt% in water) was purchased from Aldrich (Shanghai, China). AgNO3 and sodium citrate trihydrate were obtained from Shanghai Chemical Reagent Co. (Shanghai, China). All of the reagents were used as received. Glassware used in the following procedures was cleaned in a bath of freshly prepared 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HCl/HNO3 and rinsed thoroughly in Millipore water prior to use. Insulating poly(vinyl alcohol) (PVA) with an average degree of polymerization 1750 ± 50 was purchased from Ling feng Chemical reagent Co. (Shanghai, China).
1 HCl/HNO3 and rinsed thoroughly in Millipore water prior to use. Insulating poly(vinyl alcohol) (PVA) with an average degree of polymerization 1750 ± 50 was purchased from Ling feng Chemical reagent Co. (Shanghai, China).
2.2 Synthesis of AgNW–rGO hybrid
To prepare rGO, graphene oxide (GO) was first synthesized by the modified Hummers method.26,27In situ growth of 1D AgNWs on 2D PDDA-functionalized graphene sheets were obtained according to the literatures19,28,29 with some modifications. To a mixture of silver nitrate aqueous solution (0.1 M, 1 mL) and sodium citrate aqueous solution (0.1 M, 1 mL) in a flake, 10 mL of distilled water were poured. After stirring for 30 min, a homogenous and limpid solution (part A solution) was obtained. To GO aqueous (0.12 mg mL−1, 30 mL), PDDA (1.42 mL) was added. The mixture (part B solution) was sonicated for 30 min, vigorously stirred for another 1 h. Subsequently, part A and part B were mixed and stirred at room temperature for 12 h. During this process, the flake was covered with aluminum foil to prevent light-induced reduction of Ag+. The mixture was transferred into an oil bath and stirred at 130 °C for 3 h, following which 0.036 g of sodium citrate was gradually added to the mixture within 3 min. After stirring at 90 °C for 10 h, the mixture was cooled naturally to room temperature and a deep grey suspension was obtained. Solid and solution were separated by continuous washing and centrifugation at 3000 rpm for 20 min with distilled water and ethanol at least 3 times. The obtained suspension is marked as AgNW–rGO. For comparison, bare rGO was prepared using the same hydrothermal method without the addition of AgNO3 and PDDA.
2.3 Preparation of transparent conductive films (TCFs)
Samples of PVA films with various concentrations of AgNW–rGO fillers were prepared using a solution casting technique in the following way: PVA was dissolved in distilled water with a concentration of 8 wt% at 90 °C for 2 h and then degassed in an ultrasonic bath at 25 °C for 30 min. The AgNW–rGO suspensions (1.02 mg mL−1) were gradually dropped into the stirred PVA solution. The predetermined weight contents of the AgNW–rGO in the composite films range from 0.51 to 7.65 wt% respectively. The aqueous mixtures were stirred (600 rpm) at room temperature for 6 h and no aggregation was observed. The aqueous suspensions were casted onto the glass culture dish with the diameter of 47 mm and dried in an oven at 40 °C for 72 h. Then the films were detached from the glass plate, and thin films of thickness ranging from 0.1 to 0.15 mm were obtained. The thickness of the film was calculated by using a digital micrometer.2.4 Characterization
Zeta potential results were carried out on zeta potential/particle sizer (Nicomp 380ZLS, PSS corporation, America). The selected area electron diffraction (SAED) and high resolution transmission electron microscopy (HRTEM) measurements were conducted on JEM-2100F electron microscopes (JEOL corporation, Japan) operating at 200 kV with samples deposited on a carbon coated copper grid. X-ray diffraction (XRD) analysis was performed directly on the hybrid samples using a Rigaku (Japan)-Ultima IV (XRD; 40 kV, 40 mA) with Cu irradiation at a scanning rate of 0.02 s−1 in the 2θ range of 2–40°. The morphologies were characterized by scanning electron microscopy (SUPRA55, Carl Zeiss Company, German) and observed at an accelerating voltage of 3.0 kV. The sample was gold-sputtered prior to observation. The light transmittance was measured with light transmittance and haze Test System (WGT-S, Labthink Company, China) according to ASTM D1003-61(1997) at 550 nm. Raman spectra were recorded using a LabRam-1B Raman spectroscope (JY Company, French) with He–Ne laser excitation at 632.8 nm, scanning for 50 s. The electrical conductivity of the composite films was tested on RTS-9 four point resistivity test system (Anhemeng Company, China) at room temperature. Atomic force microscopy (AFM) images of the films deposited on quartz substrates were obtained with a MFP-3D (Asylum Research, America) instrument in tapping mode, using frequency of 0.803 Hz. The prepared suspension was spin-coated onto a silicon wafer to investigate the structure of the AgNW–rGO using atomic force microscopy (AFM). Digital photograph has been taken with a Canon Power Shot A590 IS 8 Megapixel camera (Japan) with 4× optical zooming.3. Results and discussion
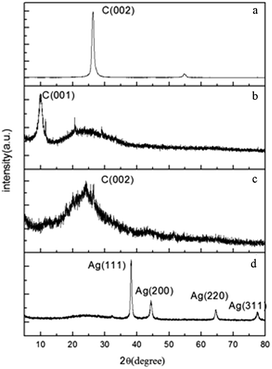
Zeta potential measurement is applied to determine the stability of aqueous graphene and carbon nanotube dispersions in previous studies,30,31 colloids with zeta potentials higher than 30 mV (negative or positive) are generally stable. The zeta potential of the aqueous GO and AgNW–rGO is −42.5 mV and 48.8 mV, respectively. As expected, the aqueous dispersion of AgNW–rGO or GO is stable and has no sign of coagulation for more than two months, while the rGO aqueous solution shows obvious precipitation (Fig. 1).Fig. 2 illustrates the XRD patterns of the graphite, GO, rGO and AgNW–rGO. Graphite (Fig. 2a) exhibits a characteristic (002) reflection peak at 26.4° with a typical interspacing of 3.35 Å, which indicates the high crystallinity of this material (JCPDS no. 41-1487). The (001) diffraction peak of the GO at 10.4° corresponding to interplanar spacing of 8.2 Å appears while (002) reflection peak of graphite at 26.4° disappears (Fig. 2b). The increased interlayer distance of the GO is attributed to the presence of the intercalated oxygen-containing groups within GO, suggesting that the graphite has been successfully oxidized by Hummers' method. A new broad diffraction peak at 2θ = 20–30° in Fig. 2b and c indicates that the successful reduction of GO32 and the disordered stacking of rGO sheets,33 respectively. The XRD pattern of the AgNW–rGO (Fig. 2d) displays four sharp peaks at 38.2°, 44.4°, 64.5° and 77.5°, which correspond to the (111), (200), (220) and (311) planes of pure fcc Ag crystals (JCPDS no. 04-0783), respectively. Furthermore, the broad (002) diffraction peak at 2θ = 20–30° appears, which is an indication that GO has been reduced to graphene and restored to an ordered crystalline structure.34
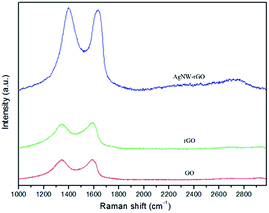
Fig. 3 shows the Raman spectra of GO, rGO and AgNW–rGO. A broad D band and G band are observed at 1350 cm−1 and 1580 cm−1, which represent the in-plane bond-stretching motion of the pairs of C sp2 atoms (the E2g phonons) and a breathing mode of A1g symmetry,35 respectively. The intensity of the D band is higher than that of the G band due to the defects and partially disordered crystal structure of GO and rGO, suggesting that the GO and rGO have a high defect content. AgNW–rGO displays an increased D/G intensity ratio (ID/IG = 1.78) compared to GO (ID/IG = 1.71), indicating a reduction in the size of the in-plane sp2 domains and a partially ordered crystal structure of AgNW–rGO.36 Additionally, the G band of AgNW–rGO red-shifts from 1588 to 1594 cm−1 relative to GO, which provides reliable evidence for the charge transfer between the graphene sheets and AgNWs. A broad 2D peak appears at a position greater than 2700 cm−1 in the AgNW–rGO Raman spectrum, indicating that the rGO is multilayer graphene nanosheet.37
Fig. 4a presents a scanning electron microscopy (SEM) image of as-prepared AgNW–rGO. It shows that the AgNWs with uniform diameter of 60 nm randomly and densely attach to the surface of graphene nanosheets, which sharply decrease the π–π and electric interactions (∼r−6) among nanosheets, therefore act as spacers to keep the neighboring nanosheets separate. Moreover, graphene nanosheets and AgNWs are decorated by large amounts of well-dispersed silver nanoparticles (AgNPs) with uniform diameter of 20 nm. The typical TEM image of the as-prepared AgNW–rGO (Fig. 4b) further demonstrates that AgNWs distributed randomly on the transparent graphene nanosheets. The HRTEM image (Fig. 4c) of AgNWs indicates that the nanowires grows along the [111] direction, and the measured interplanar spacing of AgNW is 0.235 nm, which is consistent with the spacing of the (111) planes of face-centered cubic silver crystals (JCPDS no. 87-0720; a = 4.077 Å). The selected area electron diffraction (Fig. 4d) also indicates that the as-prepared AgNWs grow predominantly along the [111] direction and are single crystal.
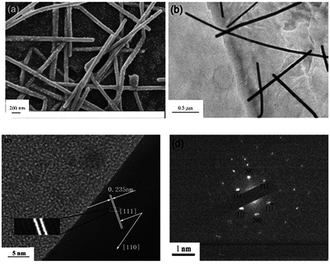 | ||
| Fig. 4 (a) SEM images of AgNW–rGO hybrids, (b) TEM images of the as-synthesized AgNW–rGO, (c) HRTEM image of a typical individual AgNW, and (d) SAED pattern of AgNW. | ||
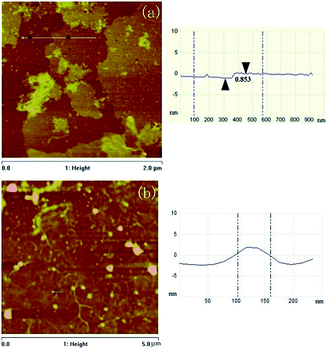
AFM images of GO and AgNW–rGO were obtained by depositing a drop of diluted dispersion on a SiO2 substrate with concentration of 0.05 mg mL−1 and drying in an ambient environment, the corresponding images are illustrated in Fig. 5. The cross-sectional view of a typical AFM image of the GO (Fig. 5a) shows that its thickness is about 0.853 nm, indicating the full exfoliation of GO. By comparison, the as-prepared AgNW–rGO has a rougher surface, and the AgNWs with diameter of about 60 nm adsorb on the surface of the graphene sheets.
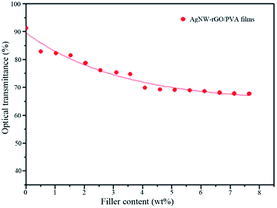
The AgNW–rGO/PVA films are very transparent and flexible (Fig. 6), and the variation of the optical transmittance with the filler content was illustrated in Fig. 7. With the filler content increase, the light transmittance decreases at first and almost has no change when the filler content ranges from 4.08 to 7.65 wt%, and 67.7% of light transmittance at 550 nm is obtained for the AgNW–rGO/PVA film containing 7.65 wt% fillers. The hybrid films seem not uniform, which is contributed to the well-known “coffee ring” effect38 that different water evaporation rates in different film positions leads aggregation of the AgNW–rGOs during the film-forming process.
 | ||
| Fig. 6 Digital photos of (a) pure PVA film, (b) 4.08 wt% AgNW–rGO/PVA film and (c) 7.65 wt% AgNW–rGO/PVA film. | ||
Electrical measurements of hybrid films with a thickness of about 0.1 mm were conducted using the four-probe technique with silver electrodes. The electrical conductivities of AgNW–rGO/PVA films and rGO/PVA films as a function of the filler content are plotted in Fig. 8. For rGO/PVA film, the conductivity increases slowly from 1 × 10−3 S m−1 to 1.67 S m−1 as rGO content increases from 0.51 wt% to 6.75 wt%. The conductivity of rGO/PVA are greater than Yang's work39 which reported that the conductivity of rGO/PVA films is less than 1 S m−1 when rGO content is less than 10 wt% and a threshold about 10 wt% of rGO was observed. Therefore, the rGO was reduced very well during the in situ preparation process.
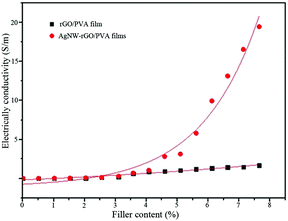 | ||
| Fig. 8 The electrical conductivity of AgNW–rGO/PVA and rGO/PVA films as a function of the filler content. | ||
For AgNW–rGO/PVA films, a low electrical percolation threshold is observed at about 4.5 wt% AgNW–rGO content, below which the conductivity keeps almost the same with rGO/PVA films and above which the conductivity increases sharply. Therefore, a conductive network may be formed when filler content is beyond 4.5 wt%. The conductivity of the AgNW–rGO/PVA film is increased by 5 orders of magnitude from 5.48 × 10−3 S m−1 to 19.5 S m−1 as the AgNW–rGO content increases from 0.51 wt% to 7.65 wt%. It is reported the conductivities of Ag/PET films prepared by vacuum filtration method range from 2 × 105 S m−1 for the 60 nm thick film to 5 × 106 S m−1 for films with thickness larger than 160 nm.40 The high performance is by virtue of excellent surface conductivity of pure AgNWs on PET films. As we know, it is the first time to form bulk AgNW–rGO/PVA conductive films by a facile preparation method. The lower conductivity of the hybrid films may be caused by the incomplete reduction of rGO and the considerable Ag nanoparticles formed simultaneously during AgNWs growth.
The competitive growth of AgNWs or AgNPs is critical to form conductive network in hybrid films. Although the mechanisms of the growth of AgNWs on rGO sheets are still not very clear, we tentatively speculate that the whole mechanism as follows: (1) at the first step, a white precipitate, silver citrate complex, is formed in part A solution upon mixing silver nitrate solution with sodium citrate solution due to its limited solubility in water (0.0285 g L−1 at 25 °C).41 At the same time, a cation polyelectrolyte PDDA is absorbed on GO surface in part B solution because of π–π and electric attraction among them, resulting good dispersion of GO sheets.42 (2) Upon mixing part A and part B, the formation of colloidal AgCl further decreases the Ag+ concentration in solution (Ksp of AgCl in water is 1.2 × 10−6 at 100 °C).41 The low Ag+ concentration is crucial for anisotropic growth of AgNWs. At the same time, a large excess of chloride ions leads to the formation of a soluble [AgCl2]− complex,43 which could act as a complex counterion for the cationic PDDA, forming ion pairs on GO sheets. After temperature is raised to 130 °C, the Ag+ is reduced to Ag and the released citrate and chloride ions are acting as capping agents to induce the formation of AgNWs41 as well as the counterions for cationic PDDA to keep AgNWs growth on the surface of GO. (3) Finally, GO is reduced by sodium citrate and the AgNWs, by functioning as a “spacer”, increase the distance between the rGO sheets, thereby impedes the formation of a stacked graphitic structure. Further studies of the factors of the concentrations of silver nitrate, sodium citrate and PDDA on the growth of AgNW–rGO will be necessary to elucidate the precise mechanisms.
4. Conclusion
In summary, we offer a convenient approach to in situ growth of AgNWs on the surface of the rGO. The AgNWs with uniform diameter of 60 nm randomly and densely attached to the rGO surface, and the aqueous dispersion of AgNWs–rGO hybrids is stable for more than two months. A high electrical conductivity of 19.5 S m−1 is achieved from transparent AgNWs–rGO/PVA films with 7.65 wt% hybrids.Acknowledgements
This work was financially funded by NSFC (Grant no. 21304012), the Natural Science Foundation of Jiangsu Province (BK20130249), the Priority Academic Program Development of Jiangsu Higher Education Institution, Jiangsu Joint Research Project of Industry, Education and Academy (KYZ13020168), and Qing Lan project of Jiangsu.References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- J. Cervenka, A. Budi, N. Dontschuk, A. Stacey, A. Tadich, K. J. Rietwyk, A. Schenk, M. T. Edmonds, Y. F. Yin, N. Medhekar, M. Kalbac and C. I. Pakes, Nanoscale, 2015, 7, 1471–1478 RSC.
- Q. B. Zheng, M. M. Gudarzi, S. J. Wang, Y. Geng, Z. G. Li and J. Kim, Carbon, 2011, 49, 2905–2916 CrossRef CAS.
- L. F. Yang, X. G. Yu, M. S. Xu, H. Z. Chen and D. Yang, J. Mater. Chem. A, 2014, 2, 16877–16883 CAS.
- V. Chabot, D. Higgins, A. P. Yu, X. C. Xiao, Z. W. Chen and J. J. Zhang, Energy Environ. Sci., 2014, 7, 1564–1596 CAS.
- Y. J. Wang, F. M. Wang and J. He, Nanoscale, 2013, 5, 11291–11297 RSC.
- X. M. Zhao and M. D. Chen, RSC Adv., 2014, 4, 63596–63602 RSC.
- X. W. Wang, L. C. Yin and G. Liu, Chem. Commun., 2014, 50, 3460–3463 RSC.
- Y. Li and N. Chopra, Phys. Chem. Chem. Phys., 2015, 17, 12881–12893 RSC.
- D. B. Yu, J. F. Yao, L. Qiu, Y. Z. Wu, L. X. Li, Y. Feng, Q. Liu, D. Li and H. T. Wang, RSC Adv., 2013, 3, 11552–11555 RSC.
- Q. Zhang, Y. F. Di, C. M. Huard, L. J. Guo, J. Wei and J. B. Guo, J. Mater. Chem. C, 2015, 3, 1528–1536 RSC.
- L. F. Shi, J. H. Yang, T. Yang, Q. Hanxu, J. Li and Q. B. Zheng, RSC Adv., 2014, 4, 43270–43277 RSC.
- S. M. Li, Y. S. Wang, S. T. Hsiao, W. H. Liao, C. W. Lin, S. Y. Yang, H. W. Tien, C. C. M. Ma and C. C. Hu, J. Mater. Chem. C, 2015, 3, 9444–9453 RSC.
- Y. Liu, Q. H. Chang and L. Huang, J. Mater. Chem. C, 2013, 1, 2970–2974 RSC.
- S. T. Hsiao, H. W. Tien, W. H. Liao, Y. S. Wang, S. M. Li, C. C. MMa, Y. H. Yu and W. P. Chuang, J. Mater. Chem. C, 2014, 2, 7284–7291 RSC.
- A. N. Sidorov, G. W. Sławiński, A. H. Jayatissa, F. P. Zamborini and G. U. Sumanasekera, Carbon, 2012, 50, 699–705 CrossRef CAS.
- S. Wageh, L. He, A. A. Al-Ghamdi, Y. A. Al-Turki and S. C. Tjong, RSC Adv., 2014, 4, 28426–28431 RSC.
- H. Zhao, H. G. Fu, T. S. Zhao, L. Wang and T. X. Tan, J. Colloid Interface Sci., 2012, 375, 30–34 CrossRef CAS PubMed.
- W. H. Yuan, Y. J. Gu and L. Li, Appl. Surf. Sci., 2012, 261, 753–758 CrossRef CAS.
- J. F. Shen, M. Shi, B. Yan, H. W. Ma, N. Li and M. X. Ye, J. Mater. Chem., 2011, 21, 7795–7801 RSC.
- C. Karuppiah, K. Muthupandi, S. M. Chen, M. A. Ali, S. Palanisamy, A. Rajan, P. Prakash, F. M. A. Al-hemaid and B. S. Lou, RSC Adv., 2015, 5, 31139–31146 RSC.
- A. M. Golsheikh, N. M. Huang, H. N. Lim and R. Zakaria, RSC Adv., 2014, 4, 36401–36411 RSC.
- S. M. Cui, S. Mao, Z. H. Wen, J. B. Chang, Y. Zhang and J. H. Chen, Analyst, 2013, 138, 2877–2882 RSC.
- J. H. Lopes, S. Y. Ye, J. T. Gostick, J. E. Barralet and G. Merle, Langmuir, 2015, 31, 9718–9727 CrossRef CAS PubMed.
- A. K. Samantara, D. K. Mishra, S. R. Suryawanshi, M. A. More, R. Thapa, D. J. Late, B. K. Jena and C. S. Rout, RSC Adv., 2015, 5, 41887–41893 RSC.
- Y. Ahn, Y. Jeong and Y. Lee, ACS Appl. Mater. Interfaces, 2012, 4, 6410–6414 CAS.
- X. Zhao, Q. H. Zhang and D. J. Chen, Macromolecules, 2010, 43, 2357–2363 CrossRef CAS.
- Z. Q. Yang, H. J. Qian, H. Y. Chen and J. N. Anker, J. Colloid Interface Sci., 2010, 352, 285–291 CrossRef CAS PubMed.
- J. Tang, D. P. Tang, B. L. Su, Q. F. Li, B. Qiu and G. N. Chen, Electrochim. Acta, 2011, 56, 8168–8175 CrossRef CAS.
- L. Chen, S. G. Chai, K. Liu, N. Y. Ning, J. Gao, Q. F. Liu, F. Chen and Q. Fu, ACS Appl. Mater. Interfaces, 2012, 4, 4398–4404 CAS.
- D. S. Hecht, L. B. Hu and G. Irvin, Adv. Mater., 2011, 23, 1482–1513 CrossRef CAS PubMed.
- S. Liu, J. Q. Tian, L. Wang, H. L. Li, Y. W. Zhang and X. P. Sun, Macromolecules, 2010, 43, 10078–10083 CrossRef CAS.
- Y. Yang, L. L. Ren, C. Zhang, S. Huang and T. X. Liu, ACS Appl. Mater. Interfaces, 2011, 3, 2779–2785 CAS.
- J. Q. Tian, S. Liu, Y. W. Zhang, H. Y. Li, L. Wang, Y. L. Luo, A. M. Asiri, A. O. Al-youbi and X. P. Sun, Inorg. Chem., 2012, 51, 4742–4746 CrossRef CAS PubMed.
- J. F. Shen, T. Li, M. Shi, N. Li and M. X. Ye, Mater. Sci. Eng., C, 2012, 32, 2042–2047 CrossRef CAS.
- Z. Zhang, F. G. Xu, W. S. Yang, M. Y. Guo, X. D. Wang, B. L. Zhang and J. L. Tang, Chem. Commun., 2011, 47, 6440–6442 RSC.
- K. H. Liu, L. Liu, Y. F. Luo and D. M. Jia, J. Mater. Chem., 2012, 22, 20342–20352 RSC.
- P. J. Yunker, T. Still, M. A. Lohr and A. G. Yodh, Nature, 2011, 476, 308–311 CrossRef CAS PubMed.
- J. H. Yang and Y. D. Lee, J. Mater. Chem., 2012, 22, 8512–8517 RSC.
- S. De, T. M. Higgins, P. E. Lyons, E. M. Doherty, P. N. Nirmalraj, W. J. Blau, J. J. Boland and J. N. Coleman, ACS Nano, 2009, 3, 1767–1774 CrossRef CAS PubMed.
- J. Q. Tang, Q. R. Zhao, N. Zhang and S. Q. Man, Micro Nano Lett., 2015, 10, 71–75 Search PubMed.
- S. Zhang, Y. Y. Shao, H. G. Liao, M. H. Engelhard, G. P. Yin and Y. H. Lin, ACS Nano, 2011, 5, 1785–1791 CrossRef CAS PubMed.
- A. B. R. Mayer, S. H. Hausner and J. E. Mark, Polym. J., 2000, 32, 15–22 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |