Fabrication of carbon-coated NiO supported on graphene for high performance supercapacitors†
Abstract
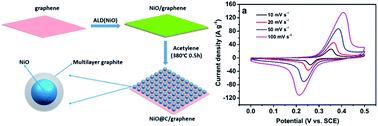
In this work, carbon-coated NiO nanoparticles supported on graphene (NiO@C/graphene) have been synthesized by integrating an atomic layer deposition (ALD) technique with a simple acetylene decomposition method. Transmission electron microscopy, X-ray diffraction analysis, X-ray photoelectron spectroscopy and Raman results demonstrated that uniform carbon films were coated onto the surfaces of NiO nanoparticles supported on graphene. The electrochemical properties of the NiO@C/graphene were then investigated. The results showed that the special design enabled synergistic effects from graphene and the carbon layer to improve the electrochemical capacitive properties of NiO. As a supercapacitor electrode, the 400-NiO@C/graphene exhibits an initial specific capacitance of 408 F g−1 (1838 F g−1 for NiO) at 1 A g−1 and 68% is retained at 50 A g−1. After 2000 charge–discharge cycles, the specific capacitance improves the initial value of ∼28% at a high current density of 10 A g−1, suggesting a great potential for high performance supercapacitors.

 Please wait while we load your content...
Please wait while we load your content...