A radical coupling reaction of DMSO with sodium arylsulfinates in air : mild utilization of DMSO as C1 resource for the synthesis of arylsulfonyl dibromomethane†
Abstract
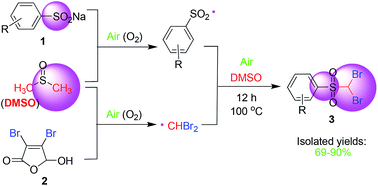
A radical coupling reaction of DMSO with sodium arylsulfinates under air atmosphere to afford arylsulfonyl dibromomethane is described. This transformation provides a novel approach for the utilization of DMSO as a C1 resource with mild temperature without the need for an anaerobic atmosphere.

 Please wait while we load your content...
Please wait while we load your content...