Systematical investigation of in vitro molecular interaction between fluorescent carbon dots and human serum albumin†
Shan Huangab,
Hangna Qiua,
Jiangning Xiea,
Chusheng Huanga,
Wei Sua,
Baoqing Hub and
Qi Xiao*ab
aCollege of Chemistry and Materials Science, Guangxi Teachers Education University, Nanning 530001, P. R. China. E-mail: qi.xiao@whu.edu.cn; Fax: +86 771 3908065; Tel: +86 771 3908065
bKey Laboratory of Beibu Gulf Environment Change and Resources Utilization (Guangxi Teachers Education University), Ministry of Education, P. R. China
First published on 28th April 2016
Abstract
Fluorescent carbon dots (CDs) have been widely applied in biological and biomedical applications due to their superior properties. Therefore, for any in vivo biomedical application, the thermodynamic and kinetic information of in vitro molecular interaction between CDs and human serum albumin (HSA) is very important and need to be elucidated deeply. In this work, in vitro molecular interaction between CDs and HSA was systematically investigated by spectroscopic techniques and electrochemical approaches. CDs with maximum emission of 437 nm were synthesized by microwave technique through a one-pot process. Some important thermodynamic and kinetic parameters were calculated, and the binding interaction between HSA and CDs was further explored by electrochemical approaches. The binding interaction of CDs with HSA was resulted from the complex formation of HSA–CDs. Hydrogen bonding and van der Waals interactions played major roles during HSA–CDs complex stabilization. The primary binding site of CDs was mainly located within site I (subdomain IIA) of HSA. The micro-environmental and conformational changes of HSA induced by CDs were investigated by multi-spectroscopic methods. These data suggested that the conformational change of HSA was significantly at secondary structure level and the biological activity of HSA was weakened dramatically in the present of CDs.
1. Introduction
As novel carbon-based nanomaterials, fluorescent carbon dots (CDs) have emerged as candidates for diverse research fields due to their superior photoluminescence features, excellent photo-electronic properties and favorable biocompatibility.1–3 These advantages make CDs to be widely applied as excellent fluorescent sensors in biological and biomedical fields, also as effective drug carriers in disease diagnosis and cancer therapy.4,5 Due to tremendous focus on biomedical applications of CDs, there has been increasing interest in understanding the molecular interaction between CDs and protein in biological systems. Compared with their bulk counterparts, CDs possess relatively higher active surface to interact with biological substances. After introducing into biological systems, some nanoparticles can interact immediately with various biological substances to form new biological entities (protein corona),6,7 which will affect the practical biomedical applications of these nanoparticles significantly. Since the original interactions of nanoparticles with biological entities, such as cells, tissues and organs, will be replaced by the serum protein layer adsorbed on the surface of nanoparticles.8 Therefore, before the sustained extensive research as well as practical biomedical applications, the thermodynamic and kinetic information of molecular interaction between CDs and serum proteins is very important and need to be elucidated urgently.9,10Human serum albumin (HSA), which is the main serum protein, participates in various important physiological functions, such as transportation, distribution, and delivery of numerous endogenous and exogenous substances.11,12 Therefore, HSA is widely selected as a model serum protein for the investigation of the interaction between nanoparticles and serum protein. Much attentions have been paid to the molecular interactions between semi-conductor quantum dots (QDs) and HSA.13–16 Xu et al. investigated the interactions between CdTe QDs with different sizes and HSA.13 In our previous studies, the binding interactions of HSA with CdSe/ZnS QDs, CdTe QDs with different sizes, and Zn-doped CdTe QDs with different sizes were investigated systematically.14–16 It has been proved that some semi-conductor QDs can bind to HSA, which in turn alters the metabolism of QDs and changes the structure of HSA. These detailed investigations on the binding affinity and the molecular interaction mechanism between different fluorescent nanomaterials and HSA are highly significant for thoroughly understanding the pharmacokinetic behavior of these fluorescent nanomaterials and deeply elucidating their molecular toxicity to organisms.
However, to our knowledge, as the assured in vivo potential of CDs in biological researches, the in vitro molecular interaction between CDs and HSA is rarely systematically involved. Recently, Liu's group investigated the interaction of their synthesized CDs with HSA.17 Their results showed that CDs did not induce any perturbation of the biological activity of HSA and the fluorescence of proteins were quenched by CDs through dynamic mechanism rather than the formation of HSA–CDs complex.17 Since CDs just collide with HSA and do not bind with HSA tightly, such collision process between CDs and HSA is not very good for effectively investigating the metabolism of CDs in organisms and deeply illuminating the biological behavior of CDs. In addition, our group indicated that GQDs could bind and alter the secondary structure of HSA significantly.18 However, different synthesis processes of carbon-based fluorescent nanomaterials may result in different structures and different photoluminescence properties of them,2,3,19,20 which will alter the interactions between different carbon-based fluorescent nanomaterials and proteins largely. Therefore, it is still necessary and quite important to systematically investigate the in vitro molecular interaction between CDs synthesized by other process and HSA for fully evaluating the biological effects of CDs on molecular biology level.
In this article, systematical investigation of in vitro molecular interaction between CDs and HSA under the approximate physiological condition was reported. CDs were synthesized by carbonization of glucose with poly(ethylene glycol)-200 (PEG-200) in microwave assisted one-pot synthesis.21 In order to analyze the interaction of CDs with HSA in detail, and to investigate the thermodynamics property of their interaction, systematical investigation and thorough understanding of HSA–CDs association was carried out by using spectroscopic and electrochemical approaches. Particularly, the conformational variations of HSA induced by CDs were further investigated by using multi-spectroscopic techniques, which may potentially predict the behavior of CDs at the nano–bio interface and greatly assist in their safe application in biomedical applications. This research may provide valuable information to the growing concerns regarding the potential toxicity risk of CDs on organisms from the molecular level, and serve as important strategy for better understanding the basic behavior of CDs in nanomedical applications and environmental exposure.
2. Materials and methods
2.1. Materials
Glucose and PEG-200 were obtained from Sinopharm Chemical Reagent. HSA, warfarin, and ibuprofen were purchased from Sigma. HSA was dissolved in 0.1 M PBS (pH 7.4) and stored at 4 °C. The concentration of HSA was determined through absorption spectrometry by using an extinction coefficient ε280 nm = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 L mol−1 cm−1.22 All other reagents were of analytical reagent grade. Ultrapure water (18.2 MΩ) was produced by Millipore-Q Academic purification set (Millipore, USA) and used throughout all experiments.
600 L mol−1 cm−1.22 All other reagents were of analytical reagent grade. Ultrapure water (18.2 MΩ) was produced by Millipore-Q Academic purification set (Millipore, USA) and used throughout all experiments.
2.2. Apparatus
UV-vis absorption spectra were measured on Cary 100 UV-vis spectrophotometer (Agilent Technologies, Inc., Australia). Fluorescence spectra were performed on Perkin-Elmer LS-55 luminescence spectrometer (PerkinElmer, USA) equipped with thermostatic bath with a 1.0 cm quartz cell. Time-resolved fluorescence spectra were recorded with Fluorolog-3 system (Horiba Jobin Yvon, France). Matrix-assisted laser desorption/ionization time of flight mass spectrum (MALDI-TOF-MS) was performed on a Bruker OmniFlex LT MALDI-TOF instrument (Bruker Daltonics Inc., USA) using 2,5-dihydroxybenzoic acid as the matrix. FT-IR spectra were recorded on Nicolet iS10 spectrometer (Thermo, USA). Circular dichroism (CD) spectra were recorded on Chirascan CD spectrometer (Applied Photophysics, England). High-resolution transmission electron microscopy (HRTEM) images were taken by Tecnai G2 F30 S-Twin FEI high-resolution transmission electron microscope (Philips-FEI, Netherlands). Cyclic voltammograms (CV) and electrochemical impedance spectra (EIS) were performed on CHI-660E electrochemical workstation (Chenhua Instruments Inc., China). All pH measurements were made with basic pH meter PB-10 (Sartorius Scientific Instruments Co., Ltd., China).2.3. Preparation of CDs
CDs were synthesized according to the literature after minor modifications.20 Briefly, 0.366 g glucose and 5.0 mL PEG-200 were mixed and dissolved into 10.0 mL water to form a clear solution. The mixture was placed in a 200 W microwave oven at 160 °C for 1 h and then a yellow CDs solution was obtained. The obtained CDs were dialyzed overnight using dialysis membranes of 500 cutoffs. Then the solution inside the dialysis membranes were dialyzed overnight using dialysis membranes of 1000 cutoffs. Finally, the solution outside the dialysis membranes was concentrated to 10.0 mL and stored at 4 °C for application. The molar concentration of CDs was measured on the basis of mean molecular weight determined by MALDI-TOF-MS.172.4. UV-vis absorption spectrometry
UV-vis absorption spectrum of CDs was measured from 200 nm to 600 nm in 0.1 M PBS (pH 7.4) at 25 °C. UV-vis absorption spectra of HSA, CDs, as well as HSA–CDs systems were measured in the wavelength range of 200–400 nm in 0.1 M PBS (pH 7.4) at 25 °C. The solutions of the blank buffer and sample were placed in the reference and sample cuvettes, respectively. The concentrations of HSA and CDs were 2.0 × 10−6 mol L−1 and 1.38 × 10−4 mol L−1, respectively.2.5. Fluorescence spectrometry
Steady-state fluorescence spectra of HSA with different concentrations of CDs were recorded at 298 K, 304 K, and 310 K in the wavelength range of 300–450 nm. The widths of the excitation and the emission slit were set to 8.0 nm and 6.0 nm, respectively. The excitation wavelength was 295 nm and the scan rate was 600 nm min−1. Each spectrum was the average of three scans. The concentration of HSA was 2.0 × 10−6 mol L−1. The concentration of CDs increased from 0 to 1.38 × 10−4 mol L−1 with an interval of 1.38 × 10−5 mol L−1.Time-resolved fluorescence spectra of HSA and HSA–CDs system were recorded at room temperature by using excitation wavelength of 278 nm and emission wavelength of 350 nm. The widths of the excitation and the emission slit were all set to 8.0 nm. The concentration of HSA and CDs were 2.0 × 10−6 mol L−1 and 1.38 × 10−4 mol L−1, respectively.
Fluorescence anisotropy measurements were performed at room temperature. Each titration point of the sample equilibration at least twenty times with an integration time of 10 min was collected. The fluorescence anisotropy of the interaction between HSA and CDs was followed at excitation wavelength of 345 nm and emission wavelength of 437 nm, respectively. The widths of the excitation and the emission slit were all set to 8.0 nm. The concentration of CDs was 3.44 × 10−5 mol L−1. The concentration of HSA increased from 0 to 3.0 × 10−5 mol L−1 with an interval of 1.0 × 10−6 mol L−1.
Three-dimensional fluorescence spectra of HSA and HSA–CDs system were performed with the excitation wavelength range of 200–350 nm and the emission wavelength range of 200–500 nm with increment of 5 nm. The scan rate was 600 nm min−1. The widths of the excitation and the emission slit were set to 9.0 nm and 8.0 nm, respectively. The concentration of HSA was 5.0 × 10−7 mol L−1. The concentration of CDs increased from 1.72 × 10−5 mol L−1 to 3.44 × 10−5 mol L−1.
2.6. FT-IR spectrometry
FT-IR spectra of HSA and HSA–CDs system were recorded in the range of 1440–1740 cm−1 with 128 interferograms and a resolution of 4 cm−1. The corresponding absorbance contributions of buffer and CDs were recorded and digitally subtracted with the same instrumental parameters. The subtraction of the reference spectrum from the spectrum of protein solution was carried out in accord with the criteria that a straight baseline was obtained between 2000 and 1750 cm−1.23 The concentration of HSA was 2.0 × 10−4 mol L−1. The concentration of CDs increased from 1.03 × 10−2 mol L−1 to 2.06 × 10−2 mol L−1.2.7. CD spectrometry
CD spectra were recorded in the range of 190–260 nm at room temperature under constant nitrogen flush. CD profiles were obtained employing a scan speed of 500 nm min−1 and response time of 0.5 s. Each spectrum was the average of three successive scans and was corrected by buffer solution. The concentration of HSA was 2.0 × 10−6 mol L−1. The concentrations of CDs were increased from 0 to 4.42 × 10−3 mol L−1.2.8. Electrochemical investigation
CV and EIS were performed with conventional three-electrode electrochemical testing system. The working electrode was CDs modified glassy carbon electrode (GCE), whereas Ag/AgCl electrode served as the reference electrode and platinum wire was used as the counter electrode. The electrolyte solution was 5.0 × 10−3 mol L−1 [Fe(CN)6]3−/[Fe(CN)6]4− and 10 mM KCl. In addition, 0.05% Tween 20 (volume ratio) was added into the electrolyte solution for elimination of nonspecific adsorption of HSA on CDs/GCE. For CDs/GCE preparation, 5 μL CDs solution (4.13 × 10−2 mol L−1) was dropped onto the surface of GCE. After 4 h incubation at room temperature, the electrode was washed by ultrapure water and then CDs/GCE was obtained. For CV and EIS measurement, different amounts of HSA solution were added continuously into the electrolyte solution with 0.05% Tween 20 and then stirred for 5 min. The reaction system was at rest for 3 min before testing. CV was recorded at a scan rate of 50 mV s−1 and EIS was measured within the frequency range from 0.1 to 100 kHz. All measurements were repeated three times with different CDs/GCE at 298 K. The concentration of HSA increased from 0 to 1.6 × 10−6 mol L−1 with an interval of 2.0 × 10−7 mol L−1.3. Results and discussion
3.1. Characterization of CDs
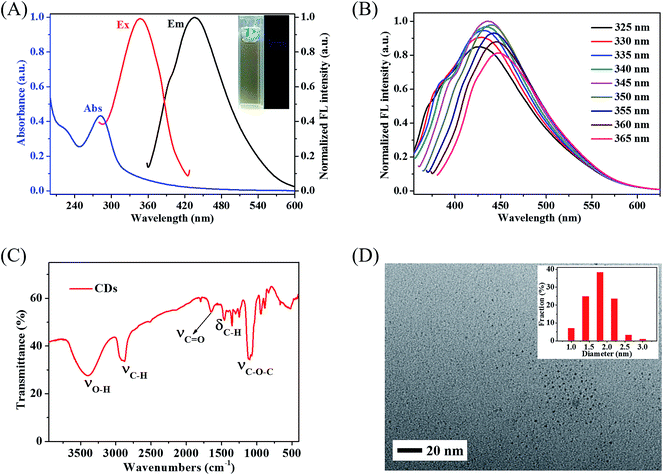
Fig. 1A displays the normalized UV-vis absorption and normalized fluorescence spectra of CDs. It was obvious that CDs exhibited broad absorption bands from 200 nm to 360 nm in the typical UV-vis absorption spectrum. Same with most carbon-based fluorescent nanomaterials, an obvious UV-vis absorption peak centered at 282 nm, which could be assigned to π–π* electronic transition of aromatic sp2 bond.17 Moreover, these CDs exhibited an obvious, narrow, almost symmetrical fluorescence spectrum with peak position of 437 nm under excitation wavelength of 345 nm, with a Stokes shift of 92 nm. From the digital pictures inserted in Fig. 1A, the diluted CDs solution was tawny under ambient daylight and exhibited slight blue emission under UV light (365 nm), which showed the weak fluorescent property of CDs. The relative quantum yield (QY) of CDs was examined to be about 1.5% under 350 nm excitation in reference to quinine sulphate whose quantum yield was 54% in 0.1 M H2SO4 solution (Fig. S1†), and the fluorescent lifetime of CDs was calculated to be around 3.15 ns (Fig. S2†), which further confirmed the low fluorescence efficiency of these CDs. In order to eliminate the inner filter effect of CDs on both the excitation wavelength and the emission spectrum of HSA, lower fluorescence efficiency of diluted CDs was chosen to research the pure molecular interaction of HSA and CDs under the approximate physiological condition.Moreover, these CDs exhibited an excitation-dependent fluorescent behavior.24 As indicated in Fig. 1B, with the excitation wavelength increased from 325 nm to 365 nm, the maximum emission peak position of CDs showed an obvious redshift continuously, but the maximum fluorescence intensity of CDs was increased firstly and decreased subsequently. These results elucidated that the maximum excitation wavelength and the maximum emission wavelength of CDs were at 345 nm and 437 nm, respectively. Such excitation-dependent fluorescent behavior was related to the different surface states of carbon-based nanomaterials.18 The stability of CDs was also investigated by monitoring of fluorescence intensity at room temperature in wide pH range (pH 3.0 to pH 10.0), and it should be pointed out that the fluorescence intensity of CDs at 437 nm changed slightly and these CDs exhibited high photostability at room temperature during wide pH range (Fig. S3†).
The surface functional groups present on CDs was identified by FT-IR spectrum. As shown in Fig. 1C, the absorption peaks at 3397 cm−1 and 2881 cm−1 were attributed to the stretching vibrations of O–H and C–H bonds, respectively. The absorption peaks at 1352 cm−1 and 1457 cm−1 were assigned to C–H bending vibrations, and the absorption peaks at 1067 cm−1 and 1101 cm−1 correspond to C–O stretching vibrations, respectively. The stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds around 1644 cm−1 was also detected. These results revealed that these CDs were equipped with –COOH and –OH groups.25 Therefore, CDs exhibited excellent water solubility and could be easily interacted with biomolecules due to the oxygen-containing functional groups. Moreover, the HRTEM images indicated that these CDs were nearly spherical with good size distribution and excellent monodispersity. The average size of CDs was around 1.8 nm (Fig. 1D). The mean molecular weight of CDs was also investigated by MALDI-TOF-MS, and the results indicated that the mean molecular weight of CDs was around 613 (Fig. S4†).
O bonds around 1644 cm−1 was also detected. These results revealed that these CDs were equipped with –COOH and –OH groups.25 Therefore, CDs exhibited excellent water solubility and could be easily interacted with biomolecules due to the oxygen-containing functional groups. Moreover, the HRTEM images indicated that these CDs were nearly spherical with good size distribution and excellent monodispersity. The average size of CDs was around 1.8 nm (Fig. 1D). The mean molecular weight of CDs was also investigated by MALDI-TOF-MS, and the results indicated that the mean molecular weight of CDs was around 613 (Fig. S4†).
3.2. Effect of CDs on HSA fluorescence spectra and quenching mechanism
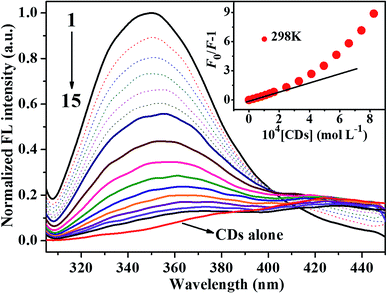
The intrinsic fluorescence of HSA is mainly ascribed to the lone tryptophan residue,26 and the fluorescence quenching efficiency of HSA by other molecules were widely studied by steady-state fluorescence techniques.15,17 In order to investigate the molecular interaction between CDs and HSA, the influence of CDs with different concentrations on the steady-state fluorescence intensity of HSA at 298 K was shown in Fig. 2. It was obvious that HSA showed strong fluorescence emission with peak position of 350 nm under excitation wavelength of 295 nm. However, CDs exhibited almost no fluorescence emission under the same conditions, which suggested that CDs could not affect the fluorescence property of HSA at all. Meanwhile, the intrinsic fluorescence intensity of HSA was quenched gradually with the increase of the concentration of CDs, which suggested that CDs quenched the intrinsic fluorescence of HSA mainly through concentration dependent manner. The concentration-dependent fluorescence change indicated the potentially greater toxicity to HSA at higher concentrations of CDs. The insert of Fig. 2 showed that the results agreed with the Stern–Volmer equation excellently at lower concentration of CDs (0 to 1.65 × 10−4 mol L−1). However, the results departed from the initial linearity at higher concentrations of CDs. In order to avoid the inner filter effects, the experiment was carried out within the linear part of Stern–Volmer dependence and the concentration of CDs was increased from 0 to 1.38 × 10−4 mol L−1.Fluorescence quenching mechanism is usually classified as static quenching and/or dynamic quenching which can be distinguished by difference dependence on temperature through fluorescence spectra and viscosity, or preferably by fluorescence lifetime detection.27 Usually, fluorescence quenching constants decrease with the increase of temperature for static quenching, since higher temperature typically results in the dissociation of weakly bound complexes. On the contrary, fluorescence quenching constants increase at higher temperature, because higher temperature results in larger diffusion coefficient and hence increased quenching efficiency. Therefore, the influences of CDs with different concentrations on the steady-state fluorescence intensity of HSA at 298 K, 304 K and 310 K were shown in Fig. S5.† Fluorescence quenching constants of HSA–CDs system at three temperatures can be calculated by the well-known Stern–Volmer equation:28
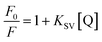 | (1) |
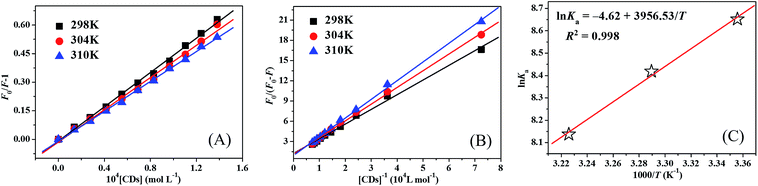
Herein, F0 and F are the fluorescence intensities of HSA in the absence and presence of CDs, respectively. KSV is the Stern–Volmer quenching constant and [Q] is the concentration of CDs, respectively. Therefore, the value of KSV can be calculated by the linear regression of the plot of F0/F against [Q] according to the upper equation. Stern–Volmer plots of HSA–CDs system at three temperatures were shown in Fig. 3A, and the calculated KSV values were listed in Table 1. It was clear that the Stern–Volmer quenching constant KSV values decreased accordingly with the temperature rising, which suggested that the fluorescence quenching mechanism of HSA–CDs system was static fluorescence quenching. Although Liu's group previously reported that their KSV values increased with increased temperature and their CDs could dynamically quench the fluorescence of HSA,17 different surface functional groups and different sizes of CDs could result in different binding modes between CDs and HSA, which were the same with other fluorescent semi-conductor nanomaterials.13,15,16 Such static fluorescence quenching mode and stable complex formation are much better for the transportation of CDs in human blood circulation.
 | ||
| Fig. 3 Stern–Volmer plots (A), modified Stern–Volmer plots (B), and van't Hoff plot (C) of HSA–CDs system. | ||
| pH value | T (K) | KSV (103 L mol−1) | R2a | S.D.b | Ka (103 L mol−1) | R2a | ΔH (kJ mol−1) | ΔG (kJ mol−1) | ΔS (J mol−1 K−1) | R2a | S.D.b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a R2 is the correlation coefficient.b S.D. is standard deviation. | |||||||||||
| 7.4 | 298 | 4.51 | 0.998 | 0.011 | 5.72 | 0.998 | −32.89 | −21.44 | −38.38 | 0.998 | 0.023 |
| 304 | 4.23 | 0.997 | 0.016 | 4.53 | 0.999 | −21.28 | |||||
| 310 | 3.93 | 0.999 | 0.007 | 3.42 | 0.999 | −20.97 | |||||
For static quenching process, the fluorescence quenching data can be analyzed to calculate the association binding constant (Ka) by the modified Stern–Volmer equation:29
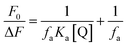 | (2) |
Herein, fa is the mole fraction of solvent accessible fluorophore.30 Therefore, the value of Ka can be calculated from the quotient of ordinate fa−1 and slope (faKa)−1 from the modified Stern–Volmer plot of F0/ΔF against [Q]−1 (Fig. 3B). The calculated Ka values of HSA–CDs system at three temperatures were also summed in Table 1. The decreasing trend of Ka with increasing temperature was in accord with KSV's dependence on temperature as discussed previously, which further demonstrated the probable static quenching mode of HSA by CDs.
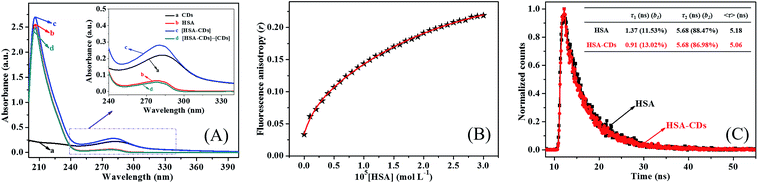
Fluorescence quenching mechanism can be illustrated further by careful examination of UV-vis absorption spectrum of fluorophore.31 It is widely reported that dynamic fluorescence quenching merely affect the excited state of fluorophore and no variations occur in the absorption spectrum of fluorophore, but static fluorescence quenching often result in the perturbation of the absorption spectrum of fluorophore.17,18 As shown in Fig. 4A, the UV-vis absorption spectrum of HSA could not overlap perfectly with the difference absorption spectrum between HSA–CDs system and CDs at the same wavelength range. These results reconfirmed the ground state complex formation of CDs with HSA and the static fluorescence quenching mechanism of HSA–CDs system.
Fluorescence anisotropy measurements can give details about binding phenomenon between protein and drugs.32 The fluorescence anisotropy can be defined by the following equations:28
| r = (IVV − GIVH)/(IVV + 2GIVH) | (3) |
| G = IVH/IVV | (4) |
Herein, G represents the instrument grating correction factor and r is the fluorescence anisotropy, respectively. IVV and IVH are the intensities obtained with the excitation polarizer oriented vertically and the emission polarizer oriented vertically and horizontally, respectively. The fluorescence anisotropy of the interaction between HSA and CDs was investigated and the results were shown in Fig. 4B. It was obvious that a marked increment in fluorescence anisotropy value with increasing concentration of HSA, which indicated that the rotational diffusion of CDs was restricted dramatically and the Brownian motion of CDs was decreased at the present of HSA with higher concentration.33 High fluorescence anisotropy value (r ≈ 0.21) of CDs after addition of HSA suggested that CDs was bound at a motional restricted site of HSA. On the one hand, these results also confirmed the ground state complex formation of CDs with HSA and the static fluorescence quenching mode of HSA–CDs system.
Time-resolved fluorescence spectrometry is an effective approach to directly clarify the exact fluorescence quenching mechanism by investigating the variation of the fluorescence lifetime of fluorophore.28 The fluorescence decay curves of HSA and HSA–CDs system were shown in Fig. 4C. The fluorescence decay curves were fitted with biexponential equation and the average fluorescence lifetime was given by the sum value of ∑τibi products.34 As exhibited in Fig. 4C, the average fluorescence lifetime of HSA alone and HSA in HSA–CDs system were about 5.18 ns and 5.06 ns, respectively. Such tiny variation of fluorescence lifetime of HSA could be omitted within experimental error. Furthermore, the fluorescence lifetime of CDs with the addition of HSA were investigated. The results showed that the average fluorescence lifetime of CDs changed slightly after the addition of HSA within the experimental error (Fig. S6†), which further proved the static fluorescence quenching mechanism of HSA by CDs.
3.3. Binding interaction between CDs and HSA
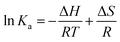 | (5) |
| ΔG = ΔH − TΔS | (6) |
In the above equations, Ka is analogous to the associative binding constant at the corresponding temperature T, and R is the gas constant, respectively. It could be seen from Fig. 3C that a good linear relationship between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka and 1/T were existed. Therefore, the values of ΔH, ΔS, and ΔG were calculated according to the linear regression equation inserted in Fig. 3C. The calculated values of ΔH, ΔS, and ΔG were all incorporated in Table 1. Negative values of ΔG suggested that the binding process of CDs and HSA were spontaneous, and both negative values of ΔH and ΔS indicated that hydrogen bonding and van der Waals interactions played major roles in the binding reaction between HSA and CDs,36 which were highly consistent with the results reported previously.18 The surface of CDs is functionalized with high content of various oxygen-containing groups which are favorable for the hydrogen bonding interaction between CDs and HSA, which is also different from the reported CDs previously.17
Ka and 1/T were existed. Therefore, the values of ΔH, ΔS, and ΔG were calculated according to the linear regression equation inserted in Fig. 3C. The calculated values of ΔH, ΔS, and ΔG were all incorporated in Table 1. Negative values of ΔG suggested that the binding process of CDs and HSA were spontaneous, and both negative values of ΔH and ΔS indicated that hydrogen bonding and van der Waals interactions played major roles in the binding reaction between HSA and CDs,36 which were highly consistent with the results reported previously.18 The surface of CDs is functionalized with high content of various oxygen-containing groups which are favorable for the hydrogen bonding interaction between CDs and HSA, which is also different from the reported CDs previously.17
Since the isoelectric point of HSA is around 4.7, solution pH plays important role in the structural stability and the surface charge of HSA.37 Therefore, the effects of pH values on the binding interaction between HSA and CDs are investigated to exclude the electrostatic interaction. The influences of CDs with different concentrations on the steady-state fluorescence intensity of HSA at three different pH values (pH 4.0, 5.0 and 7.0) were shown in Fig. S7.† The Stern–Volmer quenching constants KSV values were listed in Table 2. The Stern–Volmer quenching constants KSV kept almost constant at three different pH values, suggesting that the binding force between HSA and CDs did not contain electrostatic interaction.17 On the other hand, higher concentration of strong electrolyte may hinder the electrostatic interaction between HSA and other molecules.38 In order to facilitate the comparison of the influence of NaCl on the binding interaction between HSA and CDs, the influences of CDs with different concentrations on the steady-state fluorescence intensity of HSA in the absence and presence of 0.2 M NaCl were studied (Fig. S8†). The Stern–Volmer quenching constants KSV of HSA–CDs system in the absence and presence of 0.2 M NaCl at 298 K were analyzed by the Stern–Volmer method. The results indicated that the Stern–Volmer quenching constants KSV value remained at 4.51 × 103 L mol−1 no matter without or with 0.2 M NaCl, which also eliminated the electrostatic interaction between HSA and CDs.
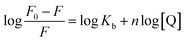 | (7) |
The values of Kb and n can be obtained by the plot of log[(F0 − F)/F] versus log[Q] (Fig. S9†). As indicated in Table 3, the value of binding constant Kb increased dramatically with the increase of temperatures, indicating that the binding capacity between CDs and HSA would be greatly increased when the temperature increased. Moreover, the binding number n was almost kept constant for HSA–CDs system in three different temperatures. These phenomenon suggested that CDs bound strongly with HSA according to mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and independent of the binding site of CDs to HSA.
1, and independent of the binding site of CDs to HSA.
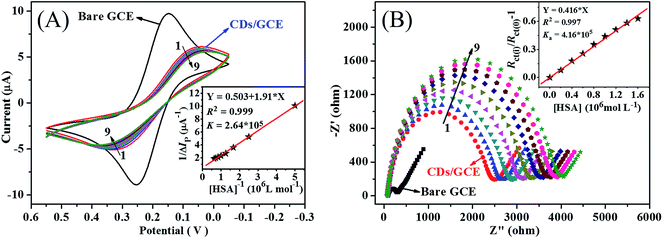
On the other hand, binding constant can be calculated through some effective electrochemical approaches. CV is an effective and widely used method to directly analyze the binding interaction between HSA and other molecules.15,18 As indicated in Fig. 6A, a good electrochemical response of [Fe(CN)6]3−/[Fe(CN)6]4− with a pair of reversible redox peaks existed on both bare GCE and CDs/GCE. However, the separation of redox peak potential increased significantly and the separation of redox peak currents decreased dramatically, showing the decrease of the electron-transfer rate of [Fe(CN)6]3−/[Fe(CN)6]4− and the conductivity of CDs/GCE. This CDs/GCE was subsequently used as the working electrode to investigate the interaction between CDs and HSA. As illustrated in Fig. 6A, when HSA was added into the [Fe(CN)6]3−/[Fe(CN)6]4− electrolyte solution, an obvious decrease in the redox peak currents and a weak shift in the redox peak potentials were happened, which ascribed to the binding interaction between HSA and CDs that reduced the diffusion coefficient of [Fe(CN)6]3−/[Fe(CN)6]4− subsequently. According to the values of the redox peak currents in the present of different concentrations of HSA, the relationship between the reciprocal of the current drop (ΔIp) and the reciprocal of HSA concentration (c) could be described with the following Langmuir equation:41
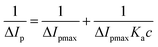 | (8) |
Herein, ΔIpmax is the maximum of the current drop and Ka is the binding equilibrium constant between HSA and CDs, respectively. As exhibited in the insert of Fig. 6A, the value of binding equilibrium constant Ka was calculated to be 2.64 × 105 L mol−1. In addition, this electrode was washed with ultrapure water three times and then dipped into fresh [Fe(CN)6]3−/[Fe(CN)6]4− electrolyte solution, the redox peak currents kept constant. These results further suggested the complex formation between HSA and CDs on the surface of GCE.
EIS was usually used to monitor the binding interaction between HSA and other molecules by comparing the value of the charge transfer resistance (Rct).15 In EIS, the value of Rct, which is represented by the diameter of the semicircle of the Nyquist plot, can be used to depict the substance modified on the electrode surface. As shown in Fig. 6B, the value of Rct increased dramatically on CDs/GCE, suggesting the successful modification of CDs on bare GCE. Furthermore, Rct values increased with the addition of HSA, which should be attributed to the increase of surface binding and the blocking of electron transfer when HSA binding to CDs/GCE. These results also suggested that HSA could bind with CDs to form the complex that increased the charge transfer resistance. Based on the changes of with the addition of HSA, the binding constant Ka could be calculated according to the equation:15
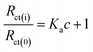 | (9) |
Herein, Rct(0) and Rct(i) represent the charge transfer resistance in the absence and presence of HSA, respectively. Therefore, the binding constant Ka can be calculated from the plot of Rct(i)/Rct(0) as a function of c. As shown in the insert illustrated in Fig. 6B, the value of the binding constant Ka was calculated to be 4.16 × 105 L mol−1. The binding constants calculated by electrochemical approaches were much higher than the binding constants obtained from spectroscopic methods. In electrochemical approaches, all possible binding might change the ΔIp value in CV and the Rct value in EIS, while the binding occurring mainly around tryptophan residue was revealed in the fluorescence titration.15,42 Therefore, the binding constants measured from CV and EIS were much higher than those measured from fluorescence spectrometry.
3.4. Conformational change investigation
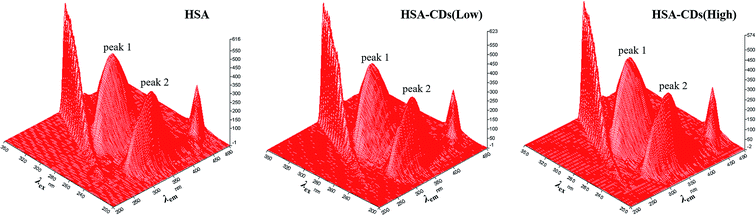 | ||
| Fig. 7 Three-dimensional fluorescence spectra of HSA and HSA–CDs system. c (HSA) = 5.0 × 10−7 mol L−1; c (CDs low) = 1.72 × 10−5 mol L−1; c (CDs high) = 3.44 × 10−5 mol L−1. | ||
| Peaks | HSA | HSA–CDs (low) | HSA–CDs (high) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak position λex/em (nm nm−1) | Stokes shift Δλ (nm) | Intensity F | Peak position λex/em (nm nm−1) | Stokes shift Δλ (nm) | Intensity F | Peak position λex/em (nm nm−1) | Stokes shift Δλ (nm) | Intensity F | |
| Fluorescence peak 1 | 290.0/346.5 | 56.5 | 485.85 | 290.0/348.1 | 58.1 | 430.10 | 290.0/348.5 | 58.5 | 396.35 |
| Fluorescence peak 2 | 235.0/346.5 | 111.5 | 412.42 | 235.0/347.1 | 112.1 | 367.07 | 235.0/348.5 | 113.5 | 346.11 |
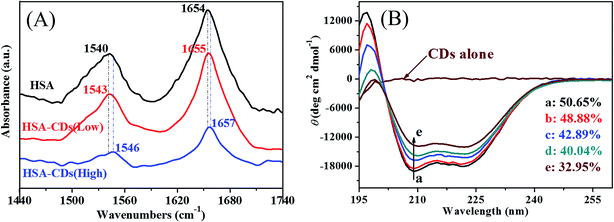
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch) and amide II band at 1540 cm−1 (C–N stretch and N–H bending mode), which were highly consistent with the reported results.43 The peak position of amide I band moved from 1654 cm−1 to 1655 cm−1 when 1.03 × 10−2 mol L−1 CDs was present, but it moved to 1657 cm−1 when 2.06 × 10−2 mol L−1 CDs was present. Meanwhile, the peak position of amide II band moved slightly from 1540 cm−1 to 1543 cm−1 (1.03 × 10−2 mol L−1 CDs) and 1546 cm−1 (2.06 × 10−2 mol L−1 CDs), respectively. These phenomena indicated that CDs affected not only the stretching vibration of C
O stretch) and amide II band at 1540 cm−1 (C–N stretch and N–H bending mode), which were highly consistent with the reported results.43 The peak position of amide I band moved from 1654 cm−1 to 1655 cm−1 when 1.03 × 10−2 mol L−1 CDs was present, but it moved to 1657 cm−1 when 2.06 × 10−2 mol L−1 CDs was present. Meanwhile, the peak position of amide II band moved slightly from 1540 cm−1 to 1543 cm−1 (1.03 × 10−2 mol L−1 CDs) and 1546 cm−1 (2.06 × 10−2 mol L−1 CDs), respectively. These phenomena indicated that CDs affected not only the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the protein polypeptides but also the C–N stretch vibration and N–H bending vibration. In general, the interaction of CDs with HSA induced the rearrangement of the secondary structure of HSA, and CDs with higher concentration exhibited greater influence on the conformational variation of HSA, which agreed well with the results obtained from fluorescence experiments.
O groups in the protein polypeptides but also the C–N stretch vibration and N–H bending vibration. In general, the interaction of CDs with HSA induced the rearrangement of the secondary structure of HSA, and CDs with higher concentration exhibited greater influence on the conformational variation of HSA, which agreed well with the results obtained from fluorescence experiments.
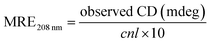 | (10) |
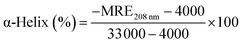 | (11) |
Herein, MRE208 is the mean residue ellipticity at 208 nm, l is the path length (0.1 cm), and n is the number of amino acid residues (n = 585 for HSA), respectively. As inserted in Fig. 8B, the α-helical content of HSA decreased from 50.65% to 42.89% or 32.95% when 2.7 × 10−3 mol L−1 or 4.42 × 10−3 mol L−1 CDs was present, respectively. The α-helical content of HSA decreased significantly after the addition of CDs with higher concentration, which suggested a stronger structural change and a lower degree of surface coverage.45 Remarkable reduction of the α-helical content of HSA in HSA–CDs system indicated the distinct conformational changes of HSA resulted from CDs. The α-helical content of HSA is related with the biological activity of HSA and the biological function of HSA will be changed due to the structure variation of HSA,46 therefore CDs with higher concentration can reduce the biological activity of HSA significantly. These conformational changes implied that HSA adopted a more incompact conformation state and an exposed hydrophobic cavities after the addition of CDs, which agreed nicely with the results obtained from fluorescence spectrometry and FT-IR experiments.
4. Conclusions
Herein, we systematically investigated the in vitro interaction between CDs and HSA. All our results indicated that the prepared CDs formed stable complex with HSA and quenched the intrinsic fluorescence of HSA by static quenching mode, which were totally different from the reported literature.17 The strong binding interaction between CDs and HSA is suitable for effectively investigating the metabolism of CDs in human and deeply illuminating the biological behavior of CDs to organisms. Besides that, higher concentration of CDs caused obvious conformational variation of HSA, indicating that the biological activity of HSA was weakened dramatically in the presence of CDs through concentration-dependent manner. These phenomena indicated the potential toxicity of CDs on organisms from the molecular level. These results make better understanding of the in vitro molecular interaction between HSA and carbon-based fluorescent nanomaterials, which is much important for the further in vivo applications of carbon-based fluorescent nanomaterials in nanomedical applications.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21563006, 21403039), Guangxi Natural Science Foundation (2013GXNSFCA019005, 2015GXNSFAA139033), Guangxi Scientific and Technological Development Projects (1598025-8), and Guangxi Colleges and Universities Key Laboratory of Synthetic and Natural Functional Molecular Chemistry, Guangxi Teachers Education University.References
- Z. H. Wang, J. B. Yu, R. J. Gui, H. Jin and Y. Z. Xia, Carbon nanomaterials-based electrochemical aptasensors, Biosens. Bioelectron., 2016, 79, 136–149 CrossRef CAS PubMed.
- L. Guo, J. C. Ge, W. M. Liu, G. L. Niu, Q. Y. Jia, H. Wang and P. F. Wang, Tunable multicolor carbon dots prepared from well-defined polythiophene derivatives and their emission mechanism, Nanoscale, 2016, 8, 729–734 RSC.
- S. Khan, A. Gupta, N. C. Verma and C. K. Nandi, Time-resolved emission reveals ensemble of emissive states as the origin of multicolor fluorescence in carbon dots, Nano Lett., 2015, 15, 8300–8305 CrossRef CAS PubMed.
- B. Wang, Y. F. Chen, Y. Y. Wu, B. Weng, Y. S. Liu, Z. S. Lu, C. M. Li and C. Yu, Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1, Biosens. Bioelectron., 2016, 78, 23–30 CrossRef CAS PubMed.
- M. Zheng, S. B. Ruan, S. Liu, T. T. Sun, D. Qu, H. F. Zhao, Z. G. Xie, H. L. Gao, X. B. Jing and Z. C. Sun, Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells, ACS Nano, 2015, 9, 11455–11461 CrossRef CAS PubMed.
- S. H. D. P. Lacerda, J. J. Park, C. Meuse, D. Pristinski, M. L. Becker, A. Karim and J. F. Douglas, Interaction of gold nanoparticles with common human blood proteins, ACS Nano, 2010, 4, 365–379 CrossRef PubMed.
- L. Treuel and G. U. Nienhaus, Toward a molecular understanding of nanoparticle–protein interactions, Biophys. Rev., 2012, 4, 137–147 CrossRef CAS.
- M. P. Monopoli, C. Åberg, A. Salvati and K. A. Dawson, Biomolecular coronas provide the biological identity of nanosized materials, Nat. Nanotechnol., 2012, 7, 779–786 CrossRef CAS PubMed.
- M. Mahmoudi, I. Lynch, M. R. Ejtehadi, M. P. Monopoli, F. B. Bombelli and S. Laurent, Protein–nanoparticle interactions: opportunities and challenges, Chem. Rev., 2011, 111, 5610–5637 CrossRef CAS PubMed.
- S. Li and N. G. Ulrich, Small fluorescent nanoparticles at the nano–bio interface, Mater. Today, 2013, 16, 58–66 CrossRef.
- Y. Zhang, Z. Q. Xu, X. R. Liu, Z. D. Qi, F. L. Jiang and Y. Liu, Conformation and thermodynamic properties of the binding of vitamin C to human serum albumin, J. Solution Chem., 2012, 41, 351–366 CrossRef CAS.
- Y. J. Hu, Y. Liu and X. H. Xiao, Investigation of the interaction between berberine and human serum albumin, Biomacromolecules, 2009, 10, 517–521 CrossRef CAS PubMed.
- Z. Q. Xu, L. Lai, D. W. Li, R. Li, C. Xiang, F. L. Jiang, S. F. Sun and Y. Liu, Toxicity of CdTe QDs with different sizes targeted to HSA investigated by two electrochemical methods, Mol. Biol. Rep., 2013, 40, 1009–1019 CrossRef CAS PubMed.
- Q. Xiao, S. Huang, Z. D. Qi, B. Zhou, Z. K. He and Y. Liu, Conformation, thermodynamics and stoichiometry of HSA adsorbed to colloidal CdSe/ZnS quantum dots, BBA Proteins and Proteomics, 2008, 1784, 1020–1027 CrossRef CAS PubMed.
- Q. Xiao, S. Huang, W. Su, P. Y. Li, J. Q. Ma, F. P. Luo, J. Chen and Y. Liu, Systematically investigations of conformation and thermodynamics of HSA adsorbed to different sizes of CdTe quantum dots, Colloids Surf., B, 2013, 102, 76–82 CrossRef CAS PubMed.
- S. Huang, H. N. Qiu, Y. Liu, C. S. Huang, J. R. Sheng, W. Su and Q. Xiao, Molecular interaction investigation between three CdTe:Zn2+ quantum dots and human serum albumin: a comparative study, Colloids Surf., B, 2015, 136, 955–962 CrossRef CAS PubMed.
- Z. Q. Xu, Q. Q. Yang, J. Y. Lan, J. Q. Zhang, W. Peng, J. C. Jin, F. L. Jiang and Y. Liu, Interactions between carbon nanodots with human serum albumin and γ-globulins: the effects on the transportation function, J. Hazard. Mater., 2016, 301, 242–249 CrossRef CAS PubMed.
- S. Huang, H. N. Qiu, S. Y. Lu, F. W. Zhu and Q. Xiao, Study on the molecular interaction of graphene quantum dots with human serum albumin: combined spectroscopic and electrochemical approaches, J. Hazard. Mater., 2015, 285, 18–26 CrossRef CAS PubMed.
- S. Huang, H. N. Qiu, F. W. Zhu, S. Y. Lu and Q. Xiao, Graphene quantum dots as on–off–on fluorescent probes for chromium(VI) and ascorbic acid, Microchim. Acta, 2015, 182, 1723–1731 CrossRef CAS.
- F. X. Wang, Z. Y. Gu, W. Lei, W. J. Wang, X. F. Xia and Q. L. Hao, Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper(II) ions, Sens. Actuators, B, 2014, 190, 516–522 CrossRef CAS.
- J. M. Liu, L. P. Lin, X. X. Wang, S. Q. Lin, W. L. Cai, L. H. Zhang and Z. Y. Zheng, Highly selective and sensitive detection of Cu2+ with lysine enhancing bovine serum albumin modified-carbon dots fluorescent probe, Analyst, 2012, 137, 2637–2642 RSC.
- G. J. Zhang, B. Keita, C. T. Craescu, S. Miron, P. D. Oliveira and L. Nadjo, Polyoxometalate binding to human serum albumin: a thermodynamic and spectroscopic approach, J. Phys. Chem. B, 2007, 111, 11253–11259 CrossRef CAS PubMed.
- A. C. Dong, P. Huang and W. S. Caughey, Protein secondary structures in water from second-derivative amide I infrared spectra, Biochemistry, 1990, 29, 3303–3308 CrossRef CAS PubMed.
- S. Huang, L. M. Wang, F. W. Zhu, W. Su, J. R. Sheng, C. S. Huang and Q. Xiao, A ratiometric nanosensor based on fluorescent carbon dots for label-free and highly selective recognition of DNA, RSC Adv., 2015, 5, 44587–44597 RSC.
- F. Y. Yan, Y. Zou, M. Wang, X. L. Mu, N. Yang and L. Chen, Highly photoluminescent carbon dots-based fluorescent chemosensors for sensitive and selective detection of mercury ions and application of imaging in living cells, Sens. Actuators, B, 2014, 192, 488–495 CrossRef CAS.
- S. Huang, F. W. Zhu, Q. Xiao, Q. Zhou, W. Su, H. N. Qiu, B. Q. Hu, J. R. Sheng and C. S. Huang, Combined spectroscopy and cyclic voltammetry investigates the interaction between [(η6-p-cymene)Ru(benzaldehyde-N(4)-phenylthiosemicarbazone)Cl]Cl anticancer drug and human serum albumin, RSC Adv., 2014, 4, 36286–36300 RSC.
- Y. Zhang and Q. X. Zhong, Probing the binding between norbixin and dairy proteins by spectroscopy methods, Food Chem., 2013, 139, 611–616 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- S. S. Lehrer, The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion, Biochemistry, 1971, 10, 3254–3263 CrossRef CAS PubMed.
- R. M. Watt and E. W. Voss, Solvent perturbation of the fluorescence of fluorescein bound to specific antibody, J. Biol. Chem., 1979, 254, 1684–1690 CAS.
- M. A. Jhonsi, A. Kathiravan and R. Renganathan, Spectroscopic studies on the interaction of colloidal capped CdS nanoparticles with bovine serum albumin, Colloids Surf., B, 2009, 72, 167–172 CrossRef PubMed.
- Q. Xiao, S. Huang, Y. Liu, F. F. Tian and J. C. Zhu, Thermodynamics, conformation and active sites of the binding of Zn–Nd hetero-bimetallic Schiff base to bovine serum albumin, J. Fluoresc., 2009, 19, 317–326 CrossRef CAS PubMed.
- A. Chakrabarty, A. Mallick, B. Haldar, P. Das and N. Chattopadhyay, Binding interaction of a biological photosensitizer with serum albumins: a biophysical study, Biomacromolecules, 2007, 8, 920–927 CrossRef CAS PubMed.
- A. Orte, J. M. Alvarez-Pez and M. J. Ruedas-Rama, Fluorescence lifetime imaging microscopy for the detection of intracellular pH with quantum dot nanosensors, ACS Nano, 2013, 7, 6378–6395 CrossRef PubMed.
- D. Leckband, Measuring the forces that control protein interactions, Annu. Rev. Biophys. Biomol. Struct., 2000, 29, 1–26 CrossRef CAS PubMed.
- D. P. Ross and S. Subramanian, Thermodynamics of protein association reactions: forces contributing to stability, Biochemistry, 1981, 20, 3096–3102 CrossRef PubMed.
- R. Weisiger, J. Gollan and R. Ockner, Receptor for albumin on the liver-cell surface may mediate uptake of fatty-acids and other albumin-bound substances, Science, 1981, 211, 1048–1050 CAS.
- S. Huang, F. W. Zhu, Q. Xiao, Y. Liang, Q. Zhou and W. Su, Thermodynamic investigation of the interaction between the [(η6-p-cymene)Ru(benzaldehyde-N4-phenylthiosemicarbazone)Cl]Cl anticancer drug and ctDNA: multispectroscopic and electrochemical studies, RSC Adv., 2015, 5, 42889–42902 RSC.
- D. E. Epps, T. J. Raub and F. J. Kézdy, A general wide-range spectrofluorometric method for measuring the site-specific affinities of drugs toward human serum albumin, Anal. Biochem., 1995, 227, 342–350 CrossRef CAS PubMed.
- S. F. Sun, B. Zhou, H. N. Hou, Y. Liu and G. Y. Xiang, Studies on the interaction between oxaprozin-E and bovine serum albumin by spectroscopic methods, Int. J. Biol. Macromol., 2006, 39, 197–200 CrossRef CAS PubMed.
- J. Zhang, R. Li, F. L. Jiang, B. Zhou, Q. Y. Luo, Q. L. Y. Yu, X. L. Han, Y. Lin, H. He, Y. Liu and Y. L. Wang, An electrochemical and surface plasmon resonance study of adsorption actions of DNA by Escherichia coli, Colloids Surf., B, 2014, 117, 68–74 CrossRef CAS PubMed.
- F. F. Tian, F. L. Jiang, X. L. Han, C. Xiang, Y. S. Ge, J. H. Li, Y. Zhang, R. Li, X. L. Ding and Y. Liu, Synthesis of a novel hydrazone derivative and biophysical studies of its interactions with bovine serum albumin by spectroscopic electrochemical, and molecular docking methods, J. Phys. Chem. B, 2010, 114, 14842–14853 CrossRef CAS PubMed.
- Y. S. Ge, C. Jin, Z. Song, J. Q. Zhang, F. L. Jiang and Y. Liu, Multi-spectroscopic analysis and molecular modeling on the interaction of curcumin and its derivatives with human serum albumin: a comparative study, Spectrochim. Acta, Part A, 2014, 124, 265–276 CrossRef CAS PubMed.
- Z. X. Lu, T. Cui and Q. L. Shi, Application of circular dichroism and optical rotatory dispersion in molecular biology, Science Press, Beijing, 1st edn, 1987, p. 79 Search PubMed.
- S. Li, Y. Z. Wang, J. G. Jiang and S. J. Dong, pH-Dependent protein conformational changes in albumin:gold nanoparticle bioconjugates: a spectroscopic study, Langmuir, 2007, 23, 2714–2721 CrossRef PubMed.
- P. Koegler, A. Clayton, H. Thissen, G. N. C. Santos and P. Kingshott, The influence of nanostructured materials on biointerfacial interactions, Adv. Drug Delivery Rev., 2012, 64, 1820–1839 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01386d |
| This journal is © The Royal Society of Chemistry 2016 |