Nano-QSAR modeling for predicting the cytotoxicity of metal oxide nanoparticles using novel descriptors†
Abstract
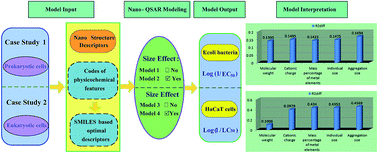
Computational approaches have evolved as efficient alternatives to understand the adverse effects of nanoparticles on human health and the environment. The potential of using Quantitative Structure–Activity Relationship (QSAR) modeling to establish statistically significant models for predicting the cytotoxicity of various metal oxide (MeOx) nanoparticles (NPs) has been investigated. A novel kind of nanospecific theoretical descriptor was proposed by integrating codes of certain physicochemical features into SMILES-based optimal descriptors to characterize the nanostructure information of NPs. The new descriptors were then applied to model MeOx NP cytotoxicity to both Escherichia coli bacteria and HaCaT cells for comparison purposes. The effects of size variation on the cytotoxicity to both types of cells were also investigated. The four resulting QSAR models were then rigorously validated, and extensively compared to other previously published models. The results demonstrated the robustness, validity and predictivity of these models. Predominant nanostructure factors responsible for MeOx NP cytotoxicity were identified through model interpretation. The results verified different mechanisms of nanotoxicity for these two types of cells. The proposed models can be expected to reliably predict the cytotoxicity of novel NPs solely from the newly developed descriptors, and provide guidance for prioritizing the design and manufacture of safer nanomaterials with desired properties.

 Please wait while we load your content...
Please wait while we load your content...