Molecular characteristics of collagen extracted from the starry triggerfish skin and its potential in the development of biodegradable packaging film†
Abstract
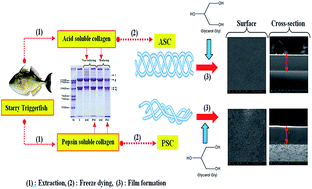
Collagen from alternative sources is being considered for various industrial applications. This study presents the extraction of collagen, a product of high value, from the skin of starry triggerfish (Abalistes stellatus). The feasibility of utilising the extracted collagens for fabrication of biodegradable food packaging film via casting method has also been investigated. The extracted acid solubilised collagen (ASC) and pepsin solubilised collagen (PSC) with yields of 7.1 ± 0.2% and 12.6 ± 0.1% (wet weight basis), respectively, were identified as type I collagen. ATR-FTIR spectra displayed that both ASC and PSC molecules had an intact triple helical structure stabilised mainly by hydrogen bonds. The net charge of ASC and PSC became zero at pHs of 5.6 and 5.4, respectively as determined by zeta potential titration. Furthermore, ASC based packaging film showed the highest tensile strength (TS), elastic modulus (E) and contact angle (θ), but the lowest elongation at break (EAB) and water vapour permeability (WVP) (P < 0.05), compared with the PSC film. Increased glass-transition temperature (Tg) and endothermic melting temperature (Tm) accompanied with higher enthalpy (ΔH) were detected in the ASC film, indicating a strong protein–protein interaction in the film network. Based on thermal analysis, the ASC film contained higher heat-stable mass residues (30.9%, w/w) as compared to the PSC film (14.3%, w/w) in the temperature range of 50–600 °C. The microstructure of the ASC film had a finer and smoother surface without layering or cracking phenomenon; however, a coarser surface was observed in the PSC film. Therefore, the skin of starry triggerfish could serve as a potential source of collagen for food packaging film applications.

 Please wait while we load your content...
Please wait while we load your content...