Designing a sulphonic acid functionalized benzimidazolium based poly(ionic liquid) for efficient adsorption of hexavalent chromium†
Avinash Ganesh Khiratkar,
S. Senthil Kumar and
Pundlik Rambhau Bhagat*
Department of Chemistry, School of Advanced Sciences, VIT University, Vellore-632014, India. E-mail: drprbhagat111@gmail.com
First published on 11th April 2016
Abstract
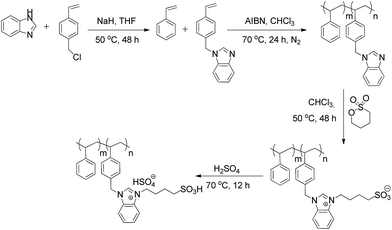
Herein, the facile synthesis of sulphonic acid functioned benzimidazolium based poly(ionic liquid) (SBPIL) is reported for the first time and we investigated its efficacy towards the removal of Cr(VI). SBPIL was synthesized by co-polymerization of styrene and 1-(4-vinylbenzyl)-1H-benzimidazole, followed by reaction with 1,4-butane sultone and subsequent functionalization with sulphonic acid. The prepared SBPIL was characterized by elemental analysis, FT-IR and NMR spectra, SEM-EDAX, TGA and X-ray diffraction techniques. The batch adsorption method was applied for examining the Cr(VI) removal and also to investigate the kinetics and thermodynamics of adsorption. The adsorption process was correlated with the Langmuir and Freundlich adsorption isotherm models and the Langmuir adsorption capacity was estimated to be 40.81 mg g−1. The thermodynamic study concluded that the adsorption process is exothermic and spontaneous and the adsorption kinetics were well fitted by a pseudo-second order kinetic model. Investigation on the effect of counter ions on the adsorption process revealed that Cl− ions had no influence on the adsorption, whereas SO42− and NO3− were found to affect the Cr(VI) adsorption by 3.6% and 19.5% respectively. SBPIL exhibited excellent stability and recyclability and the adsorbent could be easily regenerated using 1 M H2SO4 and examined for Cr(VI) removal from successive batches.
Introduction
Removal of chromium compounds is considered to be a highly preferred process since they are hazardous inorganic water pollutants causing danger to human beings and the environment by their mutagenicity and carcinogenic properties as well as causing internal hemorrhage, nausea, diarrhea, liver and kidney damage.1 Chromium compounds are released into water bodies through the effluents of industries like automobile, tannery, textile, ink manufacturing and many more.2 Cr(III) and Cr(VI) compounds mainly occur in the environment whereas the +1, +4 and +5 states are rare. Cr(III) is much less toxic than Cr(VI) and an essential element in humans. Hence, it is mandatory that wastewater containing Cr(VI) has to be treated before being released into the environment.Though various techniques such as precipitation,3 solvent extraction4,5 and activated charcoal treatment6 are investigated towards the removal of toxic metal ions, adsorption over specific solid adsorbents is highly preferred as it can be carried out with low investment and minimum space.7,8 Accordingly, adsorption process is extensively used for the treatment of industrial wastewater from organic and inorganic pollutants based on which it has attracted immense interest among researchers.9,10
In the recent past, ionic liquids (ILs) have been explored as suitable materials in water treatment owing to their high ionic conductivity, polarity and thermal stability.11,12 Moreover, the functionality (both cations and anions) of these ILs are tuneable and this makes them as interesting candidates towards the extraction of metal ions from aqueous solutions and the extraction occurs often via an ion-exchange mechanism.13–18 However, loss of ionic liquid components during the extraction process would hamper the regeneration of the ILs. In addition, some of these components such as PF6− may hydrolyze to yield toxic and corrosive products and this results in contamination of water.19,20 Though an extraction of metal ions using ILs could be achieved successfully, contamination of water by the IL components and its products are unlikely. This shortcoming could be overcome by the immobilization of ILs on a solid support and the ion-exchange characteristic of the ILs could be effectively used for efficient adsorption of metal ions.21 However, immobilization of ILs on solid support needs tedious procedures and also it is challenging to have a controlled/uniform distribution of ILs on these solid supports, which is essential for the reproducibility. Poly ionic liquids (PILs) with comparatively better processability than the corresponding monomers could be explored as suitable solid adsorbents.22 Here, the ion-exchange can occur without the leaching of any IL components into the aqueous solution. Additionally, the PILs can be made hydrophobic by metathesis with hydrophobic counter-ions and this allows easy removal of metal ions from aqueous solution by the facile heterogeneous regime.
In the present investigation, we have synthesized a sulphonic acid functionalized benzimidazolium based poly(ionic liquid) (SBPIL) and applied towards the removal of Cr(VI) ions. The structure and physicochemical properties of the SBPIL were investigated thoroughly. The kinetic and thermodynamic investigation of the adsorption was studied by examining the effect of pH, time, dose and temperature for the adsorption of Cr(VI).
Experimental
Materials
4-Vinylbenzyl chloride (90%), styrene (99.99%) and 1,4-butane sultone were purchased from Sigma-Aldrich, USA. Azobisisobutyronitrile (AIBN), sulphuric acid, hydrochloric acid, potassium chloride, sodium sulphate and potassium nitrate were obtained from Avra Synthesis, India. Sodium hydride (60% suspension in paraffin oil), potassium dichromate and chloroform were procured from S D Fine-Chem, India. Acetonitrile, tetrahydrofuran (especially dried), methanol, diethyl ether and benzimidazole were purchased from LOBA Chemie, India. A stock solution of 1000 mg L−1 was prepared by the diluting appropriate amount of potassium dichromate salt in 1000 mL milli-Q water. Working solutions were prepared by proper dilutions.Instrumentation
NMR spectra were recorded in DMSO-d6 and CDCl3 on Bruker spectrometer operating at 400 MHz and chemical shifts are given in ppm downfield from TMS (δ = 0.00). Surface morphology and the elemental composition of the adsorbent were investigated using scanning electron microscope (SEM) along with energy dispersive X-ray (EDX) spectroscopy (Carl Zeiss EVO/18SH, UK). An accelerating voltage of 10 kV was applied to obtain SEM images. The sample was coated over carbon tape and gold particles were sputtered over the sample using gold sputter coater, after which the polymeric material was directly analyzed using the scanning electron microscope. FT-IR spectra of the adsorbent were obtained from IR affinity-1 Shimadzu FT-IR spectrophotometer using KBr pellet method. Elemental analyses (C, H, N, O and S) were carried out using a EURO VECTOR EA 3000 elemental analyzer. The powder X-ray diffraction (XRD) pattern was recorded on a D8 X-ray diffractometer (Bruker, Germany) with Cu Kα radiation (λ = 1.5406 Å). Thermal stability of SBPIL was tested using TG/DTA thermoanalyser SII, 7200 (Seiko, Japan). The sample was heated from 30 °C to 800 °C and the temperature was increased at the rate of 10 °C min−1 in a nitrogen atmosphere. Thermal decomposition was taken after the weight loss of moisture from the SBPIL. Atomic absorption spectrometer (AA240, Varian, Victoria, Australia) was used to determine the unadsorbed concentration of Cr(VI).Synthesis of SBPIL
1H NMR (400 MHz, CDCl3) δ: 7.92 (s, 1H), 7.83–7.81 (d, 2H), 7.36–7.34 (d, 2H), 7.25–7.23 (d, 2H), 7.12–7.10 (d, 2H), 6.70–6.63 (dd, 1H), 5.74–5.69 (d, 1H), 5.30 (s, 2H), 5.26–5.23 (d, 1H).
13C NMR (100 MHz, CDCl3) δ: 144.02, 143.26, 137.72, 136.09, 134.90, 133.98, 127.40, 126.88, 123.19, 122.39, 120.48, 114.70, 110.13, 48.68.
1H NMR (400 MHz, CDCl3) δ: 7.81 (br, 1H), 7.21 (br, 2H), 6.95 (br, 2H), 6.73 (br, 4H), 6.38 (br, 5H), 5.09 (br, 2H), 1.94 (br, 2H), 1.60–1.13 (br, 1H). 13C NMR (400 MHz, CDCl3) δ: 144.71, 143.96, 143.15, 134, 132.82, 132.66, 128.03, 127.35, 125.85, 122.30, 120.42, 110.05, 48.47, 44.17, 40.31, 29.71, 26.32.
1H NMR (400 MHz, DMSO-d6) δ: 8.40 (br, 1H), 8.09 (br, 2H), 7.64 (br, 2H), 7.13 (br, 4H), 6.44 (br, 5H), 5.38 (br, 2H), 4.54 (br, 2H), 2.61 (br, 2H), 2.06 (br, 2H), 1.72 (br, 2H), 1.05 (br, 1H), 0.89 (br, 2H).
13C NMR (100 MHz, DMSO-d6) δ: 144.12, 143.32, 142.53, 133.61, 131.32, 130.69, 126.57, 124.59, 122.35, 121.62, 119.40, 113.91, 110.75, 50.46, 49.87, 47.49, 46.57, 45.31, 31.30, 27.62, 26.22, 22.05.
Adsorption studies
Batch adsorption experiments were performed in order to examine the adsorption capacities of the prepared SBPIL. A series of 100 mL Erlenmeyer flasks containing 20 mL of 50 mg L−1 Cr(VI) solutions and 45 mg of SBPIL were shaken at 160 rpm using an orbital shaking incubator at temperature 25 ± 1 °C for 120 min. All the adsorption studies were performed at pH 5 and the pH of the solution was adjusted using 0.1 N NaOH or 0.1 N HCl. The residual concentration of Cr(VI) was determined using flame AAS.24 The adsorption capacities of SBPIL were calculated using the following equation:
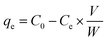 | (1) |
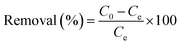 | (2) |
Results and discussion
Characterization of SBPIL and adsorption of Cr(VI) on SBPIL
The synthesized SBPIL and its adsorption capability towards Cr(VI) have been investigated using various spectral techniques. Fig. 1A shows the 1H NMR spectrum of SBPIL and the corresponding spectral data has been given in the Experimental section. The appearance of a new peak at δ 10.15 ppm corresponding to the –SO3H proton and the shifting of –CH peak of –N–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the benzimidazolium ring observed at δ 8.40 ppm in compound 3 to 9.37 ppm in compound 4, reveals that compound 3 has been successfully sulphonized to form SBPIL (4) with HSO4− counter ion.25 However, in the presence of Cr(VI), the peak at δ 9.37 ppm once again got shifted to 8.31 ppm (Fig. 1B), which shows that HSO4− counter ion has been replaced from compound 4 and hence we believe that SBPIL adsorbs Cr(VI) via the replacement of HSO4− with HCrO4−.
N in the benzimidazolium ring observed at δ 8.40 ppm in compound 3 to 9.37 ppm in compound 4, reveals that compound 3 has been successfully sulphonized to form SBPIL (4) with HSO4− counter ion.25 However, in the presence of Cr(VI), the peak at δ 9.37 ppm once again got shifted to 8.31 ppm (Fig. 1B), which shows that HSO4− counter ion has been replaced from compound 4 and hence we believe that SBPIL adsorbs Cr(VI) via the replacement of HSO4− with HCrO4−.
FT-IR spectrum of SBPILis shown in Fig. 2A which shows the characteristic broad peak for the –OH stretching frequency of –SO3H group at 3452 cm−1.26 The peaks at 2850 and 2922 cm−1 correspond to the –C–H groups of the aliphatic chain while the stretching frequency at 3057 and 3126 cm−1 is due to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H of the aromatic ring. The peaks at 615, 1035 and 1168 cm−1 correspond to the S–O symmetric vibration and S
C–H of the aromatic ring. The peaks at 615, 1035 and 1168 cm−1 correspond to the S–O symmetric vibration and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric and symmetric stretching frequency of the –SO3H respectively.27,28 In the FTIR spectrum of SBPIL after Cr(VI) adsorption (Fig. 2B), the appearance of a new peak at 938 cm−1 indicates the resonance peak of Cr–O and Cr
O asymmetric and symmetric stretching frequency of the –SO3H respectively.27,28 In the FTIR spectrum of SBPIL after Cr(VI) adsorption (Fig. 2B), the appearance of a new peak at 938 cm−1 indicates the resonance peak of Cr–O and Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and this confirms the presence of HCrO4− counter ion in SBPIL after adsorption.29
O and this confirms the presence of HCrO4− counter ion in SBPIL after adsorption.29
The variation in the surface morphology of SBPIL before and after adsorption of Cr(VI) were compared in Fig. 3A and B. Fig. 3A depicts the SEM image of synthesized SBPIL which shows a rough surface with irregularly shaped broken glass-like particles of SBPIL scattered over the surface. However, after adsorption of Cr(VI) (Fig. 3B), accumulation of tiny particles and polished surface was observed indicating the adsorption of Cr(VI) on SBPIL surface. The EDX spectrum (Fig. 3C) of SBPIL shows the presence of all expected elements in the polymer. After the adsorption of Cr(VI), new Cr peak has emerged in the region of 0.5–0.6 keV and simultaneously the intensity of S peak at 2.2–2.4 keV has significantly reduced, confirming the replacement of HSO4− by HCrO4−. Additionally, the formation of SBPIL was also confirmed using XRD and the thermal stability of SBPIL was examined using TGA (ESI, Fig. S10 and S11†).
 | ||
| Fig. 3 SEM images of SBPIL (A) before and (B) after adsorption of Cr(VI). EDX spectra of SBPIL (C) before and (D) after adsorption of Cr(VI). | ||
Optimization of experimental parameters
As SBPIL has been found to adsorb Cr(VI) ions effectively, our next step was to optimize the experimental parameters with respect to pH, adsorbent (SBPIL) dosage and contact time for achieving maximum efficiency. The effect of pH on the adsorption of Cr(VI) on SBPIL was investigated in the pH range from 2 to 10 and the results are presented in Fig. 4A. The adsorption percentage was found to increase gradually from pH 2 to 5 and was found to decrease when the pH was increased further up to 10. Generally, Cr exists in the soluble oxide forms such as H2CrO4, HCrO4−, Cr2O72− and CrO42− and the relative abundance of each species depends mainly on pH of the solution. In a highly acidic medium (pH < 2), H2CrO4 is the major species,30 and it is highly difficult for H2CrO4 to replace the HSO4− from the SBPIL and hence the adsorption efficiency was found to be very less. Upon increasing the pH from 2 to 5, the availability of HCrO4− increases and accordingly the adsorption efficiency also increased. However, beyond pH 5, Cr(VI) is available in the form of CrO42− (ref. 31) and again it becomes difficult for the CrO42− to comfortably replace the HSO4− and thus there is a sudden fall in the adsorption efficiency when the pH is increased beyond 5. This study remains as an evidence for the proposed mechanism for Cr(VI) adsorption. Since maximum adsorption was observed at pH 5, this pH was chosen as the optimum pH for further investigations. | ||
| Fig. 4 Effect of (A) pH, (B) adsorbent dosage and (C) contact time on the adsorption of Cr(VI) on SBPIL. | ||
Fig. 5B shows the adsorption efficiency of SBPIL towards Cr(VI) solutions in the presence of varying amounts of SBPIL from 25 to 55 mg. 20 mL of 50 ppm of the adsorbate used for the studies. The adsorption efficiency was found to increase upon increasing the SBPIL dosage from 25 to 45 ppm, beyond which there was no further increase. With increase in amount of the adsorbent, the number of HSO4− moieties are also expected to increase and thus favouring the adsorption of more amount of HCrO4− ions. Maximum Cr(VI) adsorption of 97.5% was achieved with 45 mg of SBPIL, and hence this amount of the adsorbent was maintained for the subsequent studies.
Similarly, the effect of contact time on the adsorption of Cr(VI) over SBPIL was also investigated (Fig. 4C). The adsorption efficiency was found to increase with time and attains the maximum at 120 min. Increase in contact time beyond 120 min, resulted in similar adsorption and thus 120 min has been chosen as the optimum contact time for the remaining studies.
Adsorption isotherm
After optimizing the experimental parameters for the adsorption of Cr(VI) on SBPIL, we were interested in understanding the adsorption behaviour. The adsorption behaviour was investigated by fitting the experimental data with Langmuir32 and Freundlich33 adsorption isotherm models.Langmuir adsorption states that adsorption of metal ions takes place on the homogeneous surface by monolayer adsorption without the interactions of adsorbed ions. The Langmuir adsorption isotherm can be expressed as
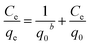 | (3) |
Freundlich adsorption isotherm assumes that the adsorption of metal ions takes place on the heterogeneous surface by monolayer adsorption. The Freundlich adsorption isotherm is expressed as
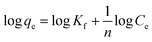 | (4) |
Upon fitting the experimental data with both Langmuir and Freundlich adsorption isotherm models, regression coefficients (R2) were found to be 0.9972 and 0.9366 respectively. The higher value of regression coefficient obtained with Langmuir adsorption isotherm indicates that the experimental data correlate well with the Langmuir model. Moreover, the maximum adsorption capacity q0 closely matches with the experimental adsorption capacity qe in Langmuir adsorption isotherm as shown in the Table 1. This result reveals that Langmuir adsorption isotherm was followed in the adsorption of Cr(VI) on SBPIL. The adsorption capacity obtained for SBPIL towards Cr(VI) adsorption is better than or comparable with the literature reports (Table 2).
| Langmuir isotherm | Freundlich isotherm | ||
|---|---|---|---|
| q0 (mg g−1) | 40.816 | Kf (mg1−1/n g−1 L1/n) | 3.3132 |
| RL | 0.0061 | n | 0.1672 |
| b (L mg−1) | 1.3528 | R2 | 0.9366 |
| R2 | 0.9972 |
| Adsorbent | Adsorption capacity (mg g−1) | References |
|---|---|---|
| Polyethylenimine facilitated ethyl cellulose | 36.8 | 36 |
| PVC–NmimCl | 23.2 | 37 |
| Cellulose ionic liquid blends polymeric material | 38.94 | 38 |
| Polymeric ionic liquid microgel beads | 74 | 39 |
| Activated carbon derived from acrylonitrile divinylbenzene copolymer | 81.516 | 40 |
| Hydrophobic poly(ionic liquid) | 17.9 | 22 |
| SBPIL | 40.81 | Present work |
Kinetics of adsorption
In order to examine the rate controlling mechanism of the adsorption process, the obtained kinetic parameters for the adsorption of Cr(VI) on SBPIL were fitted with pseudo-first order and pseudo-second order models.41,42 The pseudo-first order and the pseudo-second order equations can be expressed as follows.
log(qe − qt) = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − (k1/2.303) × t qe − (k1/2.303) × t
| (5) |
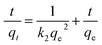 | (6) |
Further, the intra-particle rate constant was determined using the Weber and Morris equation,
 | (7) |
| Pseudo-first order kinetics | k1 (min−1) | R12 |
| 0.0138 | 0.8505 |
| Pseudo-second order kinetics | C0 (mg L−1) | qe (mg g−1) | k2 (g mg−1 min−1) | R22 |
| 50 | 24.038 | 0.0025 | 0.9887 |
| Intra-particle diffusion | kint (mg g−1 min−1/2) |
| 1.0231 |
Thermodynamic study
To investigate the thermodynamics of Cr(VI) adsorption on SBPIL, the thermodynamic parameters such as standard free energy (ΔG0), enthalpy change (ΔH0) and entropy change (ΔS0) were estimated by the following equations:
 | (8) |
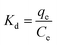 | (9) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd against 1/T (Fig. 5F). The ΔG0 values were calculated at different temperatures and the thermodynamic parameters are presented in Table 4. As indicated in the table, ΔG0 was found to be negative at all temperatures demonstrating the spontaneous and feasible adsorption of Cr(VI) on SBPIL. Furthermore, the absolute values of ΔG0 increases with the temperature, suggesting that Cr(VI) adsorption of SBPIL is favoured at high temperatures. The negative ΔH0 and ΔS0 values show that the adsorption of Cr(VI) is exothermic with decreased randomness.
Kd against 1/T (Fig. 5F). The ΔG0 values were calculated at different temperatures and the thermodynamic parameters are presented in Table 4. As indicated in the table, ΔG0 was found to be negative at all temperatures demonstrating the spontaneous and feasible adsorption of Cr(VI) on SBPIL. Furthermore, the absolute values of ΔG0 increases with the temperature, suggesting that Cr(VI) adsorption of SBPIL is favoured at high temperatures. The negative ΔH0 and ΔS0 values show that the adsorption of Cr(VI) is exothermic with decreased randomness.
| Temperature T (K) | ΔG0 (kJ mol−1) | ΔS0 (J K−1 mol−1) | ΔH0 (kJ mol−1) |
|---|---|---|---|
| 298 | −2.8917 | ||
| 303 | −3.8605 | ||
| 308 | −3.9841 | −25.3849 | −9.5624 |
| 313 | −4.4121 | ||
| 318 | −4.6647 |
Recyclability studies
In order to investigate the efficacy of SBPIL as an adsorbent in real samples, its recyclability was tested. After adsorption of Cr(VI), SBPIL could be easily regenerated using H2SO4. Cr(VI) adsorbed SBPIL was placed in the desorption medium and stirred for 180 min at 25 °C. We have carried out the Cr(VI) desorption studies using 0.1 to 1.5 mol L−1 H2SO4 and found that the maximum desorption percentage of 98% was obtained with 1 mol L−1 H2SO4. Fig. 6 shows the ability of SBPIL towards Cr(VI) adsorption for five cycles and SBPIL was recovered using 1 mol L−1 H2SO4 after every cycle. It can be observed from the Fig. 6, there is no significant change in the Cr(VI) adsorption even after five cycles and this reveals that SBPIL can be successfully recycled and reused for Cr(VI) adsorption.Effect of counter ions on Cr(VI) adsorption
In industrial waste water samples, Cr(VI) frequently co-exists with other anions like Cl−, SO4− and NO3− which may interfere with Cr(VI) adsorption. In order to examine the selectivity of the SBPIL, we have carried out the adsorption of Cr(VI) in the presence of equimolar concentrations (100 mg L−1) of the interfering anions. We observed that Cl− had no effect on Cr(VI) adsorption, whereas SO42− and NO3− ions have diminished the Cr(VI) adsorption by 0.4% and 14.4% respectively. Further, we have increased the concentration of the interfering ions (200 mg L−1) to twice that of the Cr(VI) ions. Even double the concentration of Cl− did not have any influence on Cr(VI) adsorption whereas SO42− and NO3− have reduced the adsorption by 3.6% and 19.5% respectively Fig. 7. There is no interference from Cl− which is attributed to its lower ionic radius, and thus it is difficult for Cl− ion to compete with the bulky HCrO4− in replacing the HSO4−. Though, SO42− and NO3− are large enough to replace HSO4−, they differ in their hydration energy and thus NO3− ion (with lesser ΔG0 of −314 kJ mol−1) has major interference than that of SO42− (with higher (ΔG0 = −1103 kJ mol−1).45 Also, it likely that SBPIL exhibits hydrogen bonding between C2–H of benzimidazolium cation and N of NO3−. The selectivity order of SBPIL is HCrO4− > NO3− > SO42− > Cl−.Conclusions
In this work, we have carefully designed a novel benzimidazolium containing SBPIL adsorbent for efficient removal of Cr(VI) ions from water samples. The synthesized SBPIL was found to adsorb Cr(VI) through the exchange of HSO4− of SBPIL by HCrO4− and the SBPIL could be easily recovered after Cr(VI) adsorption by treatment with 1 M H2SO4. The effect of pH, contact time and adsorbent dosage were investigated in order to optimize the operational conditions for maximum Cr(VI) adsorption. Maximum adsorption was achieved at pH 5 in 120 min in the presence of 45 mg of SBPIL. The adsorption isotherm fitted well with Langmuir isotherm and the kinetics of adsorption followed the pseudo-second order. Thermodynamic studies demonstrate that the Cr(VI) adsorption on SBPIL is spontaneous and exothermic. Furthermore, the SBPIL adsorbent exhibits excellent thermal stability, recyclability and reproducibility. This work may provide new approaches towards designing of polymer supported IL based adsorbents with highly tuneable and desirable characteristics.Acknowledgements
We gratefully acknowledge SIF DST-VIT-FIST, VIT University, Vellore for providing instrumental facilities.References
- D. Mohan and C. U. Pitman Jr., J. Hazard. Mater., 2006, 137, 762 CrossRef CAS PubMed.
- H. Gu, S. B. Rapole, Y. Huang, D. Cao, Z. Luo, S. Wei and Z. Guo, J. Mater. Chem. A, 2013, 1, 2011–2021 CAS.
- J. Esalah and M. M. Husein, Sep. Sci.Technol., 2008, 43, 3461 CrossRef CAS.
- M. Huebra, M. P. Elizalde and A. Almela, Hydrometallurgy, 2003, 68, 33 CrossRef CAS.
- D. Sevdic and H. Meider, J. Inorg. Nucl. Chem., 1977, 39, 1409 CrossRef CAS.
- M. Kobya, Adsorpt. Sci. Technol., 2004, 22, 51 CrossRef CAS.
- D. Mohan, K. P. Singh and V. K. Singh, Ind. Eng. Chem. Res., 2005, 44, 1027 CrossRef CAS.
- V. K. Gupta, A. K. Shrivastava and N. Jain, Water Res., 2001, 35, 4079 CrossRef CAS PubMed.
- F. Gao, H. Gu, H. Wang, X. Wang, B. Xiang and Z. Guo, RSC Adv., 2015, 5, 60208 RSC.
- J. Kanagaraj, R. C. panda and V. Sumathi, RSC Adv., 2015, 5, 45300 RSC.
- C. Wu, J. Fan, J. Jiang and J. Wang, RSC Adv., 2015, 5, 47165 RSC.
- O. Zech, A. Stoppa, R. Buchner and W. Kunz, J. Chem. Eng. Data, 2010, 55, 1774 CrossRef CAS.
- X. Q. Sun, H. M. Luo and S. Dai, Chem. Rev., 2012, 112, 2100 CrossRef CAS PubMed.
- A. P. de los Rios, F. J. Hernandez-Fernandez, L. J. Lozano, S. Sanchez, J. I. Moreno and C. Godinez, J. Chem. Eng. Data, 2010, 55, 605 CrossRef CAS.
- R. Germani, M. V. Mancini, G. Savelli and N. Spreti, Tetrahedron Lett., 2007, 48, 1767 CrossRef CAS.
- M. Mincher, D. Quach, Y. Liao, B. Mincher and C. Wai, Solvent Extr. Ion Exch., 2012, 30, 735 CrossRef CAS.
- P. K. Mohapatra, P. Kandwal, M. Iqbal, J. Huskens, M. S. Murali and W. Verboom, Dalton Trans., 2013, 4343 RSC.
- N. Papaiconomou, S. Génand-Pinaz, J. M. Leveque and S. Guittonneau, Dalton Trans., 2013, 1979 RSC.
- A. E. Visser and R. D. Rogers, J. Solid State Chem., 2003, 171, 109 CrossRef CAS.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, S. T. Griffin and R. D. Rogers, Ind. Eng. Chem. Res., 2000, 39, 3596 CrossRef CAS.
- L. Zhu, Y. Liu and J. Chen, Ind. Eng. Chem. Res., 2009, 48, 3261 CrossRef CAS.
- H. Mi, Z. Jiang and J. Kong, Polymers, 2013, 5, 1203 CrossRef CAS.
- P. N. Muskawar, K. Thenmozhi and P. R. Bhagat, Appl. Catal., A, 2015, 493, 158 CrossRef CAS.
- M. I. B. Toledo, J. R. Utrilla, R. O. Perez, F. C. Marin and M. S. Polo, Carbon, 2014, 73, 338 CrossRef.
- R. Kore, T. J. Dhilip Kumar and R. Srivastava, J. Mol. Catal. A: Chem., 2012, 360, 61 CrossRef CAS.
- Zillillah, G. Tana and Z. Li, Green Chem., 2012, 14, 3077 RSC.
- M. Vafaeezadeh and M. M. Hashemi, Chem. Eng. J., 2014, 250, 35 CrossRef CAS.
- A. Pourjavadi, S. H. Hosseini, M. Doulabi, S. M. Fakoorpor and F. Seidi, ACS Catal., 2012, 2, 1259 CrossRef CAS.
- M. M. Hoffmann, J. G. Darab and J. L. Fulton, J. Phys. Chem. A, 2001, 105, 1772 CrossRef CAS.
- Z. Ren, D. Kong, K. Wang and W. Zhang, J. Mater. Chem. A, 2014, 2, 17952 CAS.
- T. S. Anirudhan, S. Jalajamony and P. S. Suchithra, Colloids Surf., A, 2009, 335, 107 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40(9), 1361 CrossRef CAS.
- H. M. F. Freundlich, Z. Phys. Chem., 1906, 57, 385 CAS.
- G. Crini, H. N. Peindy, F. Gimbert and C. Robert, Sep. Purif. Technol., 2007, 53, 97 CrossRef CAS.
- S. Kalidhasan, K. K. A. Santhana, V. Rajesh and N. Rajesh, J. Hazard. Mater., 2012, 213–214, 249 CrossRef CAS PubMed.
- B. Qui, J. Guo, X. Zhang, D. Sun, H. Gu, Q. Wang, H. Wang, X. Wang, X. Zhang, B. L. Weeks, Z. Guo and S. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 19816 Search PubMed.
- M. L. Chen, Y. N. Zhao, D. W. Zhang, Y. Tian and J. H. Wang, J. Anal. At. Spectrom., 2010, 25, 1688 RSC.
- S. Kalidhasan, K. K. A. Santhana, V. Rajesh and N. Rajesh, J. Colloid Interface Sci., 2012, 367, 398 CrossRef CAS PubMed.
- M. T. Rahman, Z. Barikbin, A. Z. M. Badruddoza, P. S. Doyle and S. A. Khan, Langmuir, 2013, 29, 9535 CrossRef CAS PubMed.
- D. Duranoglu, A. W. Trochimczuk and U. Beker, Chem. Eng. J., 2012, 187, 193 CrossRef CAS.
- H. Y. Shan, Scientometrics, 2004, 59, 171 CrossRef.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451 CrossRef CAS.
- M. H. Entezari, N. Ghows and M. Chamsaz, J. Phys. Chem. A, 2005, 109, 4638 CrossRef CAS PubMed.
- E. Bulut, M. Özacar and I. Ayhan Sengil, Microporous Mesoporous Mater., 2008, 115(3), 234 CrossRef CAS.
- L. Zhu, Y. Liu and J. Chen, Ind. Eng. Chem. Res., 2009, 48, 3261 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and FTIR spectra, XRD pattern and thermogram of SBPIL, 1-(4-vinylbenyl)-1H-benzimidazole. See DOI: 10.1039/c6ra01219a |
| This journal is © The Royal Society of Chemistry 2016 |