Solution-processed MoSx thin-films as hole-transport layers for efficient polymer solar cells†
Abstract
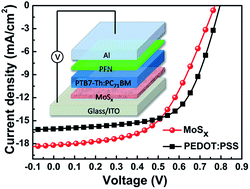
Here we employed a simple solution-processed method to prepare MoSx films as hole-transport layers (HTLs) in polymer solar cells (PSCs). Impressive performance was achieved with a power conversion efficiency (PCE) of 7.50%, which is comparable to those of the conventional devices with PEDOT:PSS HTLs. The surface and optoelectronic characteristics of MoSx films obtained from different conditions were investigated. The results reveal that the annealing temperature of MoSx is one of the most important factors on determining the device performance. PSCs based on MoSx films exhibited much better device stability compared to the counterpart based on PEDOT:PSS, suggesting that the MoSx film is a promising alternative HTL for PSCs.

 Please wait while we load your content...
Please wait while we load your content...