Designing a single superabsorbent for separating oil from both layered as well as micron/submicron size emulsified oil/water mixtures by gamma radiation assisted grafting†
Subhendu Ray Chowdhury*a,
Atanu Jhaa,
Uttam Mannab and
K. S. S. Sarmaa
aRadiation Technology Development Division, Bhabha Atomic Research Centre, Trombay, Mumbai – 400 085, India. E-mail: rcsubhen@barc.gov.in; rcsubhendu@gmail.com; Tel: +91-22-2788-7347
bDepartment of Chemistry, Indian Institute of Technology, Guwahati 400086, India
First published on 2nd March 2016
Abstract
Separation of oil–water from either its layered mixtures or emulsions is an extremely important challenge in this modern era. Commercially available polyurethane (PU) sponge does not have selectivity towards liquid. In this article, we introduce a rapid, single step, scalable, economic and sustainable route to introduce super selectivity towards oily liquid to the sponge upon modification via gamma radiation assisted grafting of a low surface energy molecule (dodecyl 2-methacrylate). The covalent bond formed through grafting process, provides a highly durable special wettable property (superhydrophobicity and superoleophilicity) to the material without compromising its inherent mechanical property. We demonstrate that single the ‘super’-oil-absorbent (modified PU sponge) is highly efficient to separate quickly both layered oil–water mixtures and emulsions (micron and submicron size), which is unprecedented in the literature. Here, the reported material provides an energy efficient and more convenient approach to separate oil–water from both layered and emulsified oil/water mixture. SEM image indicates the formation of a rough surface on a modified PU sponge with some micron, submicron and nanosize hemispheres or bumps (ups and downs) due to the gamma-radiation based grafting of DMA, which is the cause behind this transformation. Moreover, the same piece of this modified PU sponge can be repetitively used in separation of oil–water for more than 100 times at least without compromising its mechanical & physical (special wetting) properties.
Introduction
In the last few years, contamination of our precious water resources by oil spillage from improper management of industrial waste, sinking ships and frequent oil spilling accidents, has become one of the most severe environmental and ecological issues.1,2 So there is an urgent need for the development of suitable materials for collection and separation of harsh organic pollutants (mostly oily materials) from contaminated water. The techniques, which are being used to clean up such leakages or spillages are mainly skimming, and the use of chemical dispersants and sorbents.1–4 The most common and traditional approach of oil–water separation, skimming, is highly time and energy consuming and expensive in nature. Whereas chemical dispersant approach can easily separates oil into droplets, however, this method adds additional toxic chemical (various surfactants) into water and requires additional separating techniques.3,4 As another promising alternative, various oil-sorbent materials provide a meaningful and convenient approach to clean and recollect oily substance from contaminated water.5,6 Most effective sorbents so far reported are the sponge and foam based materials because of their easy availability, mechanical flexibility, reusability, low cost, very low weight to volume ratio and extremely high porosity to absorb oil in a large scale.7 However, poor selectivity of such sorbents towards oily or aqueous liquids is keeping this class of materials sidelined from potential applications in removing and collecting oil from contaminated water.In recent past, a tremendous interest is growing in the industrial and academic research related to super-anti-wetting materials for oil/water separation.8–13 The work reported here is mostly motivated by recent promising progress in oil/water separation using materials those are with special wettability (such as superhydrophobic/superoleophilic and/or superhydrophilic/superoleophobic). Common approaches to separate oil–water are performed by mostly filtration of oil–water mixture and generally involve suitable super-anti-wetting coatings (mostly, featured soft polymeric or metal oxide coatings) on various materials such as fabric, metallic mesh and carbon based material.8–13 However, these proof of concept studies are successfully demonstrated in various laboratories—but, often, poor chemical and physical durability of such coatings may restrict its immense potential in oil–water separation in practical settings (scratching, bending, squeezing, under high flux of water etc.). In recent past, few super-anti-wetting coatings with suitable and appropriate physical and chemical durability are illustrated in oil/water separation study in literature. But selection of substrates (metal mesh, fabrics and membranes) and/or approach (first collection then separation by gravity driven filtration using super-anti-wetting coated materials or anti-wetting membranes) of oil–water separation restrict the direct onsite utility of those coated materials in many occasions, where collection and transportation of contaminated water is either time consuming, inconvenient, and/or more expensive process. Moreover, a common approach to separate oil and water from oil/water emulsion generally involves gravity driven filtration of emulsion solution through either anti-wetting coated materials or anti-wetting membranes. However, this approach is useful and widely practiced in literature. But separation of oil and water from surfactant-stabilized micro/sub-micron-emulsions (having droplet size ≤ 20 μm) commonly faces severe problem of low flux and fast depreciation in permeation due to plugging of pores of the membranes or meshes by emulsion droplet, as expected, due to gradual lose in anti-wetting property of the material by regular absorption of the surfactants (that are present in emulsions) from surfactant contaminated aqueous medium over the time. However, recently, some reports are successfully dealing with emulsified oil/water separation using robust special wettable materials such as membranes (PVDF membrane, PAA-g-PVDF membrane, mussel-inspired polymer membrane), self-standing films (single-walled carbon nanotube network) and modified fabrics. These robust materials are prepared by following a special and comparatively complex fabrication process.14–18 Moreover, mechanical properties of most of these materials have not been examined in details, even though, high mechanical strength is highly desired, and more likely, most of the proposed materials are susceptible to face failure before large scale gravity driven separation of oil–water from emulsions at practical settings.
Surface wettability of a given liquid on a solid surface (or materials) can be tailored by providing suitable chemical composition and appropriate topography (degree and geometry of roughness) to the solid surface.19–21 Thus, high selective absorption (super selectivity towards a certain liquid) can be adopted on a sponge by construction of befitted surface. Some proofs of studies have already been introduced in literatures to alter the sponge surface wettability, such as in situ growth,22 dip coating,23 polymerized octadecylsiloxane coating,24 carbon nanotube/poly(dimethylsiloxane) based coating,25 block copolymer (BCP) grafting,26 solution-immersion,27 sugar templating,28 vapor phase polymerization,29 fluoroalkylsilane (FAS) modification,30 polytetrafluoro ethylene (PTFE) deposition followed by silane (octyltrichlorosilane) modification by a vapor phase deposition31 etc. Most of these existing modifications are involved in non-covalent deposition of corresponding coatings on native sponge following relatively troublesome techniques. Durability of such coating on native sponge substrates is poor and coatings are likely to be fall apart on practical scenarios such as bending, scratching, squeezing due to week and non-covalent interfacial interaction between native sponge and deposited coatings on it. Even some studies have been conducted on modification of PU sponge by troublesome chemical grafting of some special molecules.32–34 But the grafting and morphologies generated on PU surfaces are not expectedly suitable thus leading to poor performance. Moreover, the focus is only on layered oil/water separation just by generating superhydrophobic surface. Superoleophilicity and oil/water separation from emulsions are not considered in these cases. Nevertheless, these all reported special wettable sponges have only been involved in separation of layered oil/water mixture. Efficient separation of oil–water from micro-emulsion using such system is yet to explore as per authors' knowledge. Thus, despite of these continuous efforts, fabrication of a durable, onsite usable, highly efficient and reusable selective oil-absorbent material to separate oil–water from both layered mixtures and emulsions (surfactant free and surfactant stabilized) by a facile, inexpensive, environmental friendly and scalable process is highly desired and is still remained as an extremely challenging and exciting research topic.
Here, in this study, porous polyurethane (PU) sponge is strategically selected to provide essential surface chemistry and necessary physical topography by one step covalent grafting of a suitable low surface energy small molecule (dodecyl 2-methacrylate) onto the sponge substrate by applying a rapid, easily controllable, economic, worker friendly, scalable and sustainable process, gamma radiation assisted grafting. We have followed similar kind of technique of gamma radiation assisted grafting as we have mentioned in our previous publication.35 This unique single step modification process provides both essential topography and low surface energy same time, and the modified material (PU sponge) adopts extremely high selective liquid (oil/water) wetting property without affecting its native mechanical property. The PU sponge surface turns into a superhydrophobic and superoleophilic surface. Here, this reported work validates existing principle of layered oil–water separation with physically and chemically durable modified PU sponge, and broadens prospective and promising application of this class of materials in separation of oil–water from emulsions through selective absorption of emulsion droplets. This material is superhydrophobic and superoleophilic, thus it repels water and attracts oil from emulsions consequently demulsifying emulsion droplets, which leads to separation of oil and water. Moreover, we demonstrate further that our material remains highly efficient to separate oil–water even after large numbers (100 times) of repetitive uses without compromising its inherent mechanical property.
Experimental
Materials
Dodecyl 2-methacrylate (DMA), ethanol (absolute alcohol) and ethyl acetate were purchased from Sigma-Aldrich. Used polyurethane (PU) sponge was commercial sponge with 30 mg mL−1 density.Grafting of dodecyl 2-methacrylate (DMA) on PU sponge
Dodecyl 2-methacrylate (DMA) was grafted on surface of PU (polyurethane) sponge by gamma radiation in water/ethanol solution by the direct method following a similar kind of strategy mentioned in our previous publication.35 4 cm × 4 cm × 4 cm PU sponges were immersed in four different 200 mL solutions (water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol 20
ethanol 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180), which contain 20 mL, 40 mL, 50 mL and 60 mL DMA in water/ethanol solutions respectively (total volume is 200 mL). After 3 hours, the samples were irradiated by a total dose of 20 kGy using a 60Co radiation source in gamma chamber (GC-5000, BRIT, MUMBAI, INDIA). To remove the excess DMA, formed oligomers and homopolymers, the sponge blocks were washed with ethyl acetate in ultrasonic bath for 3 hours followed by 72 hour washing by the same solvent keeping the sponges immersed into it. The sponges were dried at 60 °C for 5 h.
180), which contain 20 mL, 40 mL, 50 mL and 60 mL DMA in water/ethanol solutions respectively (total volume is 200 mL). After 3 hours, the samples were irradiated by a total dose of 20 kGy using a 60Co radiation source in gamma chamber (GC-5000, BRIT, MUMBAI, INDIA). To remove the excess DMA, formed oligomers and homopolymers, the sponge blocks were washed with ethyl acetate in ultrasonic bath for 3 hours followed by 72 hour washing by the same solvent keeping the sponges immersed into it. The sponges were dried at 60 °C for 5 h.
The degree of grafting (G%) was calculated gravimetrically using the equation-
| % Grafting = (Wg − Wo)/Wo × 100, |
Preparation of the oil/water emulsions
Surfactant-free and surfactant stabilized oil/water emulsions were prepared by mixing oil (namely, toluene, dichloromethane, chloroform and mobil) and water in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 volume ratio and sonicated at a power of 2 kW for 2 h to produce white milky solutions. For surfactant stabilized oil/water emulsions, the used surfactant was 2 g/1 L TWEEN 20 (polysorbate 20).
7 volume ratio and sonicated at a power of 2 kW for 2 h to produce white milky solutions. For surfactant stabilized oil/water emulsions, the used surfactant was 2 g/1 L TWEEN 20 (polysorbate 20).
Dynamic light scattering (DLS) measurements confirmed the droplet sizes of those emulsions to be in micron to submicron range for surfactant free emulsions and submicron to nano range for surfactant stabilized emulsions. All the surfactant-stabilized oil/water emulsions were stable for more than 6 weeks, no demulsification or precipitation was observed.
Characterization
For FTIR study Bruker-Alpha's Platinum ATR model instrument was used, samples were characterized at Attenuated Total Reflection (ATR) mode at room temperature (25 °C) and wave number ranging from 400–4000 cm−1. The number of scans and spectral resolution were set to 24 s and 4 cm−1, respectively.Morphologies of pure and grafted PU surfaces were investigated by a scanning electron microscope (SEM) using JEOL, JSM-5400 model. Samples were gold coated to make the surfaces conductive. Contact angles of oil and water on pure as well as modified PU surface were measured by ramé-hart instrument standard goniometer, model no. 260-G1 (ramé-hart instrument co., Succasunna, NJ, USA). We used deionized water (drop size of 3 μL) for this experiment, and all the experiments were performed at a constant temperature (25 °C). Sony cyber shot digital camera was used for recording optical images. For XRD analysis, Philips X-ray diffractometer instrument was used with CuKα radiation source, at an operating voltage of 40 kV and current of 30 mA.
Differential scanning calorimetric (DSC) analysis was carried out with 10 °C min−1 heating and cooling rate in N2 atmosphere using DSC-131, Setaram Instrumentation, France.
Tensile testing was carried out using a universal testing machine, Tinius Olsen, Model no. HTE-1000N at a crosshead speed of 50 mm min−1 at 25 °C. Sample dimension was 65 mm × 20 mm × 10 mm. The average of four replicas is reported here.
Particle size analyzer VASCO 3, Cordouan, France was used for particle size analysis of emulsions and Olympus BX 53 was used for optical microscopy study of the emulsions.
Results and discussion
The radiation-based approach of grafting various functional moieties on wide range of polymers involves a clean, rapid, more convenient and scalable process with minimum chance of unwanted contamination, unlike conventional chemical grafting. Thus, it is appearing as a leading and routine process for suitable modification of desired material.Here, current report is involved in designing a highly oily liquid selective absorbent using radiation based grafting process to develop an efficient and economic oil–water separation from both layered and emulsified oil/water mixtures. So, a commercially available, highly porous polymer matrix, called polyurethane (PU) sponge is strategically selected to modify with dodecyl 2-methacrylate (DMA) at 20 kGy gamma radiation in water/ethanol (see Experimental section for further details). Possible covalent grafting of DMA on PU is schematically represented in Scheme 1. The grafting is confirmed by FTIR spectral analysis of both pure PU and modified PU as shown in Fig. 1A. The characteristic peaks for pure PU substrate appear at 1712 cm−1, 2865 cm−1, 2971 cm−1 and 3270 cm−1 corresponding to urethane carbonyl, –CH2 stretching, sp2 –CH stretching and N–H stretching respectively.36,37 In the grafted PU, two peaks appear at 1720 cm−1 and 2921 cm−1 corresponding to carbonyl and the –CH3 vibration,36,37 the later being present in DMA only. The shifting of carbonyl peak by 8 cm−1 (due to contribution of DMA's carbonyl) and appearance of –CH3 vibration stretching peak (2921 cm−1) in the modified PU sponge clearly suggests successful grafting of DMA onto PU sponge. The quantitative estimation of this radiation based grafting is performed following conventional gravimetric analysis. Detailed mathematical equation and calculation procedure is provided in Experimental section. Different weight percentages (18 wt%, 25 wt%, 29 wt% and 34 wt%) of DMA grafting are observed on the PU sponge based on feeding compositions of DMA and native PU substrate as discussed in Experimental section.
 | ||
| Scheme 1 Schematic illustration showing gamma-radiation based chemical modification of pure PU (polyurethane) sponge by DMA (dodecyl 2-methacrylate) molecules through ‘C–C’ covalent bond formation. | ||
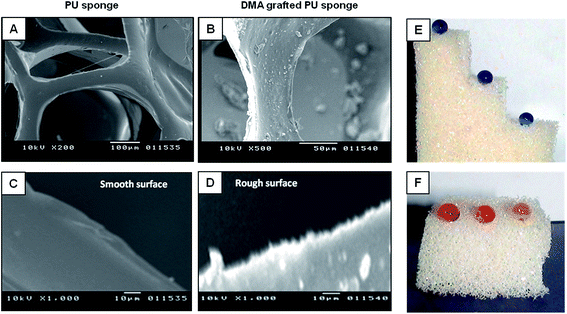
Next, we examine the effect of gamma radiation assisted grafting on the morphology of the PU sponge. Overall morphology of the pure PU remains intact upon grafting of DMA onto PU sponge as confirmed by SEM study (Fig. 1B and C). Porosity and ‘fiber-like’ micro features, present in pure PU sponge (Fig. 1C) and DMA grafted (25 wt% of grafting) PU sponge (Fig. 1B), appears mostly identical under scanning electron microscope. However, additional nano/submicron scale features are observed in modified PU sponges. This nano/submicron scale features play crucial role in adopting selective and special wetting property to the material. The nano/micron-scale morphological changes in modified PU will be further discussed later in more details. Then, anti-wetting property of the modified PU is explored with conventional water droplet beading test, static contact angles of the beaded liquid water droplets (volume of 3 μL) are measured on modified PU sponge. We notice that value of static water contact angle of modified PU sponge depends on the degree of grafted DMA. Water contact angle on modified PU sponge having 18 wt% DMA grafting is 140° ± 2°, whereas water contact angle enhances to 153° ± 2° on increasing the grafting percentage of DMA to 25 wt%. However, insignificant change in water contact angle is observed on further increase in the grafting percentages (29 wt% and 34 wt%). So, further all other studies are carried out with 25 wt% DMA grafted sponge unless otherwise mentioned. Thus, modified PU sponges with ≥25 wt% of DMA grafting display desired superhydrophobicity, water droplets are extremely repelled by the modified sponge and beaded on the modified sponge with high water contact angle (153° ± 2°) as shown in Fig. 2A and C. However, oil droplet readily soaked into the modified sponge with 0° oil contact angle on beading the oil droplet (volume 3 μL, mobil, density 872 mg mL−1) as shown in Fig. 2B and D. So, modified sponge shows desired extreme selective wetting of liquid water (>150°) and oil (∼0°), unlike pure PU sponge. Pure PU shows very poor selective wetting of both the aqueous and oily liquids with a water contact angle of 95° ± 2° and oil contact angle 70° ± 3° as shown in Fig. 2E–H. Thus, commercial sponge without any selectivity, adopts superselectivity towards oil and super disliking towards water through gamma radiation assisted DMA grafting.
This single step modification of PU sponge using gamma radiation technique does not only provide essential surface chemistry to the materials—but required nano/submicron-scale surface morphology is also developed during this grafting process as confirmed by detail surface imaging study with scanning electron microscopy (SEM). SEM image of pure PU sponge shows that surfaces of each ‘fiber-like’ domains are featureless and smooth as shown in Fig. 3A. However, SEM image in Fig. 3B indicates the formation of rough surface on modified PU sponge with some micron, submicron and nano size hemisphere or bumps (ups and downs). These features are developed on the modified sponge surface due to gamma-radiation based grafting of DMA. The sizes of hemispheres are distributed in long range from few hundreds of nanometres to few microns, and the distances between hemispheres are in the micron and submicron range as shown in Fig. S1A and B.† This topography resembles with lotus leaf.38
We hypothesize that these hemispherical morphology is developed due to deposition of oligo-DMA and or poly-DMA on native PU matrix. Gamma radiation assisted grafting is proceeded through free radical reaction. Most likely, either some post-grafted DMA molecules convert to its oligomer and polymer or some oligo-DMA and poly-DMA are post-grafted onto the PU matrix leading to construction of these essential features. This DMA-based micron, submicron and nanoscale hemispheres (Fig. S1A and B† and 3B) are amorphous in nature as confirmed by DSC and XRD study (data not shown here).
Pure PU sponge displays both moderate hydrophobicity (water contact angle ∼ 95°) and oleophilicity (oil contact angle 70°) as the surface tension value of the solid substrate (here pure PU sponge) is in-between the water and oil, according to the Young equation.19 Thus, pure PU sponge has very poor selective wetting property for liquid oil and water. As mentioned before, highly selective wetting property can only be adopted to desired substrate by combination of appropriate surface roughness and surface energy. Several theoretical and experimental studies have recently revealed the importance of topography feature behind designing of a bio inspired (lotus leaf, insect legs and wings) artificial superhydrophobic surface.38 Horizontal view of a portion of the ‘fiber-like’ domain in both the untreated- and modified-PU sponges are presented in Fig. 3C and D for better understanding of the existence of nano/submicron scale features in modified sponge. Surface of the ‘fiber-like’ structures in unmodified-PU sponge is again noticed to be smooth and featureless (Fig. 3A and C). This micron to nano-scale roughness in modified-PU sponge surface only allows heterogeneous wetting of liquid water on the modified-sponge surface, in combination with low surface energy (provided by long hydrocarbon chain containing grafted-DMA).38 However, the superoleophilicity, on the other hand, is facilitated by the low surface energy from same long chain hydrocarbon of grafted-DMA on the modified-PU sponge. Thus, both superhydrophobicity and superoleophilicity have simultaneously been adopted in the modified-PU sponge to meet desire selective wetting of both liquid oil and water. Moreover, our synthetic approach has high scalability, we have successfully modified pure sponge having large dimension (6 inches × 6 inches × 2 inches) as shown in Fig. S2,† and currently, further progress is going on to modify much larger size of PU sponge in our commercial gamma chamber (BRIT, Mumbai, India) plant.
Before exploring our modified PU sponge in oil–water separation study, some relevant physical and chemical durability tests are performed following standard protocols. A piece of modified PU sponge is subjected for oil absorption followed by manual bending and squeezing for collection of oil. This operation is done for 100 times by applying moderate pressure to examine the functional integrity of the DMA-grafted PU sponge. Superhydrophobic property of the modified material is found to be unperturbed even after 100 times bending and squeezing as the liquid water droplet is beaded with high contact angle (CA 149 °C) on this treated PU sponge surface as shown in digital images of Fig. S1C.† SEM images in Fig. 1B and in 3B clearly indicate that each ‘fiber-like’ domain (that are present in deferent depth) of PU sponge is with hemispherical feature, which is arising due to radiation-based DMA-grafting. Special wetting property of the modified sponge at different depth is further examined by measuring liquid water contact angle at three selected layers of the DMA-grafted PU sponge. Modified PU sponge (20 mm height) is sliced to expose inner layers prior to measure liquid water contact angles. The variation in liquid water contact angle at the inner layers (150° ± 2° and 149° ± 1°, as shown in Fig. S3A and B,† respectively) and at the outermost layer (153° ± 2°) of modified-PU sponge is negligible. Existence of desired anti-wetting property at entire part of the modified sponge is further revealed by digital image in Fig. 3E, where all the 10 μL aqueous (crystal violet solution, 0.1 mg mL−1 concentration) droplets are beaded with almost spherical shape on both the outermost surface (far left-top portion in Fig. 3E) and the inner layers (middle, and right-bottom portion in Fig. 3E) of the modified-PU sponge. Later, chemical durability of the modified-PU sponge is examined by exposing the material to extremes of pH and ionic strength, keeping in mind its prospective applications in oil–water separation or oil-spills cleaning in marine or in other harsh environments. Droplets of acidic water (far right, HCl, pH 2), alkaline water (far left, NaOH, pH 10) and seawater (middle) are beaded on modified-PU sponge with almost spherical shapes with minimum contact area between beaded liquid water droplet and modified sponge surface as shown in Fig. 3F (photos are taken after 20 min of beading of droplets), and thus, it suggests the existence of chemically robust anti-wetting property in DMA-grafted PU sponge.
Chemically and physically durable DMA-grafted PU sponge is then explored in oil–water separation process. As already mentioned before, our material is designed for absorption-based (a more convenient and easy approach) oil–water separation, and preliminary studies (which are described earlier) are suggesting high selective wetting of oil on modified PU sponge surface. Here, comparatively more appropriate and relevant experiment is designed further to compare the selective absorption of liquids (oily from oil/aqueous mixture) by our modified PU sponge and native PU sponge. A pieces of modified- and native-PU sponges having similar dimension (2 cm × 2 cm × 2 cm, 0.25 g) are exposed to similar layered oil/water systems (10 mL of oil and 35 mL of water) in two different beakers as shown in Fig. 4A–D. Left (modified-sponge) and right (pure-sponge) of each panel in Fig. 4A–D are showing strikingly different oil-absorbance by modified- and pure-PU sponge with time. On exposing the modified-PU sponge to layered oil/water systems, oil keeps on being absorbed into the materials with time, dragging the sponge inside the oil. The sinking and oil absorption of the modified sponge are shown at 0, 3, 5 and 7 min as shown in Fig. 4A–D. It is seen that sponge is consistently dragged into the oil and the amount of oil absorbed is consistent suggesting prompt absorption of oil by modified sponge.
A significantly large portion of the modified sponge has soaked most of the layered oil at 7 min exposure (Fig. 4D) unlike pure-PU sponge (no such noticeable change is observed for pure-PU sponge as shown in right panels of Fig. 4A–D). On extending the exposure time, right after 7 min, the immersed portion of the modified sponge has absorbed the layered oil at it's maximum capacity (8.2 mL) and has got settled on top of the water layer as shown in left panel of Fig. 4D. Whereas pure-PU sponge remains floating on oil layer and hardly any change is observed for pure-PU sponge in layered oil/water system over the exposure time. We have carried out a kinetic study of this oil-absorption by modified-sponge. The data are plotted in Fig. 4E. 8.2 mL of oil (which is almost equal of the volume of immersed portion of sponge) is recovered from the system in 7 min as shown in Fig. 4E. No significant change is observed further with time, as the sponge touches the interface of oil–water and does not go down any more. Thus, radiation based DMA-grafted PU sponge with superoleophilicity and superhydrophobicity is observed to be a remarkable oil absorbent material with high selectivity.
Moreover, modified-PU sponge is then exposed to various other oils and organic solvents having different surface tensions to estimate selected liquids uptake capacities by modified PU sponge. We have chosen seven different kinds of oils (mobil, motor oil, mustard oil, diesel and petrol) and organic solvents (toluene and chloroform). The oil uptake capacities of modified PU from layered oil (or organic solvent)/water systems are tested and results are provided in g g−1 scale in Table 1. The oil-absorption capacity of the modified porous sponge with respect to oil varies from 3000% to 6000% of absorbent's weight. The variation in densities of the tested liquids is one of the major contributors towards this large window of the absorption capacity of the modified-PU sponge. However, overall absorption capacity is remarkably high for potential and successful oil–water separation.
| Oil type | Absorption by modified PU (g g−1) | Surface tension (dyne per cm) |
|---|---|---|
| Mobil | 34.8 | 30.2 |
| Motor oil | 36 | 31.0 |
| Edible oil (mustard oil) | 36.4 | 20.1 |
| Diesel | 35 | 25.8 |
| Petrol | 30 | 24.1 |
| Toluene | 34.4 | 28.4 |
| Chloroform | 60 | 27.1 |
Here, we first demonstrated a straight-forward and more convenient absorbance based oil-removal process from layered oil/water mixture using our modified sponge, and absorbed oils are then collected in desired place by just squeezing the selective oil-absorbed material as shown in Fig. 4F–I. A piece (∼4.5 cm × 3 cm × 2 cm) of modified-PU sponge is placed in layered oil/water mixture (30 mL oil and 50 mL water). It is observed that grafted sponge is highly selective to absorb only oil from layered oil/water mixture, as expected. Later, absorbed oil is collected in desired place by simple squeezing as shown in Fig. 4I, and the amount of collected oil by this approach is estimated to be nearly same (28 mL) to the amount taken (30 mL) to prepare layered oil/water mixture. This selective oil absorption process is inherently rapid due to presence of highly porous structures with selective wetting property in modified-PU sponge. It provides opportunity to nearly complete removal and collection of oil and ‘oil-like’ liquids by exposing appropriate amount (can be roughly pre-calculated from its absorption capacity) of modified-PU sponge and followed by simple squeezing.
To investigate separation of the emulsified oil/water mixture using our modified sponge, some surfactant free and surfactant stabilized oil/water emulsions with a wide droplet size distribution ranging from nanometres to microns, are prepared (detail procedure is given in Experimental section). Modified-PU sponge (4 cm × 4 cm × 4 cm) is first immersed into the milky-white oil/water emulsions (not stabilized with surfactant) and left for 10 min. Then the immerged-sponge is collected back from the oil/water emulsions, and this process results in clean water (is almost free from contaminated oil droplets) as shown in Fig. 5A–C. A milky white emulsion solution (digital image in Fig. 5A) with wide size distribution of oil droplets (confirmed by DLS measurements) (Fig. 5B) is observed to be clean (Fig. 5C) and free from oil droplets as confirmed from optical microscope study (Fig. S4A–D†). A very similar result is noticed in case of surfactant-stabilized oil/water emulsion, though it needs longer immersion duration. The modified-sponge should be kept in contact with surfactant stabilized emulsion for 40 min to get clean water as shown in Fig. 5D–F.
We have conducted optical microscopy study (Fig. S4A–D†) as well as dynamic light scattering (DLS) study for droplet size analysis of emulsion before and after separation. DLS shows that the emulsion solution before separation comprises of micron to submicron particles (approx. up to 0.3 micron) (Fig. 5B and E). But after separation, for both cases (without and with surfactant), DLS does show error signal due to crossing of lower limit of machine sensitivity. Our particle size analyser (VISCO3), DLS can detect particle size (diameter) ranging from 0.5 nm to 10 μm and sample concentration ranging from 0.1 ppm – 40% w/v.
Thus, the studied emulsion mixtures (after separation) certainly posses negligible droplets. We have obtained in optical microscopy study (Fig. S4B and D†) almost blank pictures for both emulsions (without and with surfactant) after separation, that supports the above observation from DLS.
For the above two cases (emulsions without and with surfactants) most likely, oil/water emulsion droplets are demulsified in the vicinity of the modified sponge and thus oils are spontaneously absorbed in modified-PU sponge. If the mechanism of demulsification of oil–water in this system is expressed in a simple way, then we can put it like this: due to superhydrophobicity, the sponge repels the water strongly and for superoleophilicity sponge attracts the oil strongly. These two forces act on emulsion droplets in opposite direction. This is probably the main factor of demulsification of oil–water emulsions. Thus, when emulsion droplets come into the vicinity of modified surface demulsification takes place.14 Demulsification consists of two steps, flocculation (aggregation, agglomeration, or coagulation) and coalescence.39,40 Due to these above applied forces, the mobility of droplets is probably increased thus enhancing the droplet collisions, somewhat similar to electrostatic field application technique of demulsification.40 That probably increases the flocculation and coalescence eventually leading to demulsification. For detail mechanism more studies are required.
Lack of presence of any oil droplets in post treated emulsion solutions is confirmed by digital image (Fig. 5F). Optical microscope study suggests an extremely high separation efficiency of the modified sponge (Fig. S4A–D†). In our approach, absorbed oil is then collected back by squeezing the oil-loaded sponges. Removal of submicron and nano-scale oil droplets along with micro droplets from emulsions is another uniqueness of our approach as separation of submicron and nanosize emulsion droplets using membranes and meshes is known to be difficult task.17
Modified-PU sponge is then explored in repetitive use for oil–water separation from both the layered mixture and emulsions. Oil-soaked modified-PU sponge, right after separation of oil–water from layered and emulsified mixtures, is squeezed hard to extract out the absorbed oil to recollect the separated oil. After 100 cycles the sponge is washed thoroughly with dichloromethane and followed by air drying to remove out every trace of oil to obtain the correct value of static contact angle of water. Modified-PU sponge is found to be highly efficient to restore its anti-wetting property after air-drying the washed modified-PU sponge as suggested by contact angle measurements. The static water contact angle is found to be 149° (Fig. 6B) after 100 repetitive use in oil–water separation and the material is noticed to be equally efficient to separate oil–water from layered and emulsified oil/water mixtures. For practical use, oil-soaking and squeezing are enough, no need to wash the sponge by solvent mentioned above after every use. To get the consistent and reproducible static contact angles we have followed all these steps as mentioned above.
 | ||
| Fig. 6 (A) Stress–strain profiles of pure, modified and modified after 100 times used PU sponge. (B) Water contact angle on modified sponge after 100 time repetitive uses in oil–water separation. | ||
We hypothesised that most harsh step that may compromise the mechanical property of the material is the recollection of absorbed oil by squeezing the modified-PU sponge. So, we examined the mechanical properties of the material and compared the property before and after 100 times repetitive uses with native material. So, pure sponges, grafted sponges and 100 times used grafted-sponges are subjected to tensile testing. It is obvious from Fig. 6A that after using for 100 cycles, the sponge is found to show almost no change in mechanical properties. The stress–strain graphs of unmodified sponge, modified sponge and 100 times used sponge are almost similar. The sustainable superhydrophobicity in the material is observed due to strong carbon–carbon covalent bond between DMA and PU surface.
Conclusions
This current report accounts for the fabrication of a highly oily liquid selective absorbent by gamma assisted low surface energy molecule grafting on commercially available polyurethane sponge. The route of modification provides essential chemical (low surface energy) and physical (featured topography) requirements to adopt a highly durable special wettable (superhydrophobic and superoleophilic) property to the material without affecting the native mechanical property of the PU sponge, which is retained even after 100 times use. Modified sponge having highly selective absorbance property towards oily liquids and severe disliking towards water, provides energy efficient, rapid, scalable, reusable, economic and more convenient approach to separate oil–water from both layered and emulsified (surfactant free and surfactant stabilized) oil/water mixtures. Moreover, our material has enormous potential in dealing with many oil contamination problems, such as oil spill cleaning from water, purification of crude oil and treatment of commercially relevant emulsified solutions.Notes and references
- C. H. Peterson, S. D. Rice, J. W. Short, D. Esler, J. L. Bodkin, B. E. Ballachey and D. B. Irons, Science, 2003, 302, 2082 CrossRef CAS PubMed.
- J. Schaum, M. Cohen, S. Perry, R. Artz, R. Draxler, J. B. Frithsen, D. Heist, M. Lorber and L. Phillips, Environ. Sci. Technol., 2010, 44, 9383 CrossRef CAS PubMed.
- E. B. Kujawinski, E. B. Kido Soule, M. C. Valentine, D. C. L. Boysen, A. K. Longnecker and K. Redmond, Environ. Sci. Technol., 2011, 45, 1298 CrossRef CAS PubMed.
- V. Broje and A. A. Keller, Environ. Sci. Technol., 2006, 40, 7914 CrossRef CAS PubMed.
- H. M. Choi and R. M. Cloud, Environ. Sci. Technol., 1992, 26, 772 CrossRef CAS.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. J. Kokot, J. Porous Mater., 2003, 10, 159 CrossRef CAS.
- B. Wang, W. Li, Z. Guo and W. Liu, Chem. Soc. Rev., 2015, 44, 336 RSC.
- L. Feng, Z. Y. Zhang, Z. H. Mai, Y. M. Ma, B. Q. Liu, L. Jiang and D. B. Zhu, Angew. Chem., Int. Ed., 2004, 116, 2012 CrossRef PubMed.
- Z. X. Xue, S. T. Wang, L. Lin, L. Chen, M. J. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270 CrossRef CAS PubMed.
- H. Zhou, H. X. Wang, H. T. Niu, G. Lin and T. Robust, Adv. Funct. Mater., 2013, 23, 1664 CrossRef CAS.
- Z. G. Guo, F. Zhou, J. C. Hao and W. M. Liu, J. Am. Chem. Soc., 2005, 127, 15670 CrossRef CAS PubMed.
- X. Gui, J. Wei, K. Wang, A. Cao, H. Zhu, Y. Jia, Q. Shu and D. Wu, Adv. Mater., 2010, 22, 617 CrossRef CAS PubMed.
- C. H. Lee, N. Johnson, J. Drelich and Y. K. Yap, Carbon, 2011, 49, 669 CrossRef CAS.
- W. B. Zhang, Z. Shi, F. Zhang, X. Liu, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2071 CrossRef CAS PubMed.
- Z. Shi, W. B. Zhang, F. Zhang, X. Liu, D. Wang, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2422 CrossRef CAS PubMed.
- H. C. Yang, K. J. Liao, H. Huang, Q. Y. Wu, L. S. Wan and Z. K. Xu, J. Mater. Chem. A, 2014, 2, 10225 CAS.
- C. Yang, J. K. Pi, K. J. Liao, H. Huang, Q. Y. Wu, X. J. Huang and Z. K. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 12566 Search PubMed.
- W. Zhang, Y. Zhu, X. Liu, D. Wang, J. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed., 2014, 53, 856 CrossRef CAS PubMed.
- X. J. Feng and L. Jiang, Adv. Mater., 2006, 18, 3063 CrossRef CAS.
- K. S. Liu, M. Y. Cao, A. Fujishima and L. Jiang, Chem. Rev., 2014, 114, 10044 CrossRef CAS PubMed.
- N. Gao and Y. Yan, Nanoscale, 2012, 4, 2202 RSC.
- B. Wang, J. Li, G. Y. Wang, W. X. Liang, Y. B. Zhang, L. Shi, Z. G. Guo and W. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 1827 CAS.
- J. Wu, N. Wang, H. Dong, Y. Zhao and L. Jiang, ACS Appl. Mater. Interfaces, 2012, 4, 3207 CAS.
- Q. Ke, Y. Jin, P. Jiang and J. Yu, Langmuir, 2014, 30, 13137 CrossRef CAS PubMed.
- C. F. Wang and S. J. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 8861 CAS.
- L. B. Zhang, Z. H. Zhang and P. Wang, NPG Asia Mater., 2012, 4, e8, DOI:10.1038/am.2012.14.
- Q. Zhu, Q. M. Pan and F. T. Liu, J. Phys. Chem. C, 2011, 115, 17464 CAS.
- S. J. Choi, T. H. Kwon, H. Im, D. I. Moon, D. J. Baek, M. L. Seol, J. P. Duarte and Y. K. A. Choi, ACS Appl. Mater. Interfaces, 2011, 3, 4552 CAS.
- X. Y. Zhou, Z. Z. Zhang, X. H. Xu, X. H. Men and X. T. Zhu, Ind. Eng. Chem. Res., 2013, 52, 9411 CrossRef CAS.
- X. Zhang, Z. Li, K. Liu and L. Jiang, Adv. Funct. Mater., 2013, 23, 2881 CrossRef CAS.
- G. H. Jiang, R. B. Hu, X. G. Xi, X. H. Wang and R. J. Wang, J. Mater. Res., 2013, 28, 651 CrossRef CAS.
- V. O. A. Tanobe, T. H. D. Sydenstricker, S. C. Amico, J. V. C. Vargas and S. F. Zawadzki, J. Appl. Polym. Sci., 2009, 111, 1842 CrossRef CAS.
- H. Li, L. Liu and F. Yang, Mar. Pollut. Bull., 2012, 64, 1648 CrossRef CAS PubMed.
- J. Wang and G. Geng, Mar. Pollut. Bull., 2015, 97, 118 CrossRef CAS PubMed.
- S. Ray Chowdhury and S. Sabharwal, J. Mater. Chem., 2011, 21, 6999 RSC.
- C. Kavakl, P. A. Kavakl and O. Guven, Radiat. Phys. Chem., 2014, 94, 111 CrossRef.
- Y. Feng and C. F. Xiao, J. Appl. Polym. Sci., 2006, 101, 1248 CrossRef CAS.
- V. Zorba, E. Stratakis, M. Barberoglou, E. Spanakis, P. Tzanetakis, S. H. Anastasiadis and C. Fotakis, Adv. Mater., 2008, 20, 4049 CrossRef CAS.
- H. B. Bradley, Petroleum Engineering Handbook, Society of Petrolium Engineers, Richardson, Texas, 1987 Search PubMed.
- L. L. Schramm, Emulsions: Fundamentals and Applications in the Petroleum Industry, American Chemical Society, Washington, DC, 1992 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional characterization of modified PU sponges and 100 times used sponge by SEM study and digital picture (Fig. S1), digital images of bigger modified sponge (for scaling up) (Fig. S2), water contact angle on various layers of the modified sponge (Fig. S3) and optical microscopic pictures (Fig. S4A–D). See DOI: 10.1039/c6ra01162d |
| This journal is © The Royal Society of Chemistry 2016 |