Naringin ameliorates radiation-induced hepatic damage through modulation of Nrf2 and NF-κB pathways†
Abstract
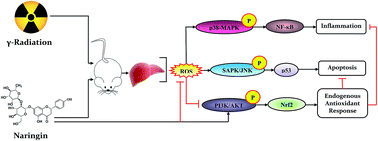
Understanding the mechanism of ionizing radiation (IR)-induced systemic stress and its modulation by phytocomponents has great significance for the development of novel phyto-radioprotectors. The present study was intended to evaluate the radioprotective effect of naringin (NG), a citrus flavonoid on the modulation of IR-induced activation of the redox-regulated signaling system in murine liver. On the basis of survival analysis, mice were treated with 75 mg kg−1 body weight of NG for three consecutive days before irradiation (6 Gy). Pretreatment with NG significantly prevented the IR-induced generation of intracellular reactive oxygen species (ROS), along with the formation of hepatic thiobarbituric acid reactive substances (TBARS) and cellular nitrite. NG also showed significant reduction in IR-induced nuclear DNA damage through the inhibition of DNA-dependent protein kinase (DNA-PK). Further, the IR-induced cell death was arrested in the presence of NG through the inhibition of p53 mediated stress-activated protein kinase/Jun amino-terminal kinase (SAPK/JNK) pathways and modulation of other molecules of apoptosis pathways. Moreover, NG supported the intracellular defense mechanisms, by maintaining the endogenous antioxidants probably through phosphoinositide 3-kinase/protein kinase-B (PI3K/Akt) guided transcriptional activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). In addition, NG inhibited IR-induced inflammation through suppression of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) followed by the alteration of pro inflammatory factors. Taken together, these results suggested that NG reversed the IR-induced redox-imbalance in murine liver, probably by the inhibition of ROS/p38-MAPK/NF-κB, along with the activation of the PI3K/Akt/Nrf2 pathway and the reduction of apoptosis by interfering with the p53/SAPK/JNK/Bax pathway.

 Please wait while we load your content...
Please wait while we load your content...