Application of novel multi-cationic ionic liquids in microwave assisted 2-amino-4H-chromene synthesis†
Arjun Kumbhar*a,
Sanjay Jadhavb,
Rajendra Shejwalc,
Gajanan Rashinkarb and
Rajshri Salunkhe*b
aDepartment of Chemistry, Padmabhushan Dr Vasantraodada Patil College, Tasgaon, Sangli, 416312, Maharashtra, India. E-mail: arjun2win@yahoo.co.in
bDepartment of Chemistry, Shivaji University, Kolhapur, Maharashtra, India
cDepartment of Chemistry, L.B.S. College, Satara, Maharashtra, India
First published on 28th January 2016
Abstract
Novel multi-cationic ionic liquids containing a mesitylene backbone with acetate and methane sulphonate anions have been synthesized. These ionic liquids were used for the synthesis of 2-amino-4H-chromenes under microwave heating. The effects of nature and amount of ionic liquids on the yield and reaction time were thoroughly investigated. The ionic liquids showed a considerable level of reusability without a significant decrease in catalytic activity. We have successfully combined the advantages of microwave technology with ionic liquids to facilitate the rapid construction of chromene skeletons from readily obtainable and inexpensive materials via a multicomponent strategy.
Introduction
Multi-component reactions (MCRs) play an important role in modern synthetic chemistry. As MCRs generally occur in a single pot, exhibit a high atom economy and good selectivity, they provide a powerful tool towards the synthesis of diverse and complex compounds as well as small heterocycles.1 Molecules with the chromene structure constitute one of the most interesting class of compounds in organic chemistry due to their biological and pharmacological importance such as antimicrobial,2 antiviral,3 antiproliferation,4 antitumor5 and central nervous system activities.6 These compounds are also employed in cosmetics, pigments and used as potential biodegradable agrochemicals.7 Generally, 2-amino-4H-chromenes are synthesized by heating aldehydes, malononitrile and phenols in presence of organic bases like piperidine in organic solvents8 and also by several modified procedures using Triton B,9 Phase Transfer Catalysts (PTCs),10 γ-alumina,11 Preyssler type heteropolyacid (H14[NaP5W30O110]),12 K2CO3,13 TiCl4,14 p-toluenesulfonic acid,15 nanostructured diphosphate Na2CaP2O7,16 and nanosize MgO.17 Due to the environmentally benign nature of electro-organic synthesis,18 Elinson et al.19 reported the electrocatalytic chain procedure for the preparation of 4H-chromenes by the combined electrolysis of salicylaldehydes and alkyl cyanoacetates in ethanol in an undivided cell. In order to avoid some of the drawbacks of reported methods, the discovery of a new and efficient catalyst with high potential, short reaction time, recyclability and simple workup procedure is highly desirable.The research in the field of ionic liquids (ILs) has grown exponentially over the last few decades due to their environmentally friendly nature, non-volatility, recyclability, thermal stability and easy workup.20 One of the most attractive features offered by IL is both the cationic and anionic components can be varied and modified so that liquid properties can be tailored for specific applications. This modification of ILs can result in unique solvent properties that can dramatically influence the outcome of various reactions. The multi-cationic ILs are superior to mono-cationic ILs as they provide more opportunities to tune their physical and chemical properties. Conventional synthesis of IL is complicated and often suffers from halogen impurities. Hydroxide based ILs now offers the simplest synthetic tool for synthesize large number of halogen free ILs. An exchange reaction of the acid with an aqueous hydroxide solution of ILs affords the desired “Task Specific Ionic Liquids (TSILs)”.
Since last few years the microwave heating becomes one of the widely used alternative technique to carry out organic transformations efficiently.21 Due to the ionic character, IL absorb microwave radiations extremely well and transfer of energy is quick by ionic conduction. The transfer of energy is more efficient with increase in temperature. Hence, when ILs are coupled with MW they exhibit dramatic effect on rate enhancement due to synergistic couple.22 In view of the emerging importance of the ILs as reaction media and our general interest in microwave as an energy source for chemical processes,23 we decided to build up a new class of mono, bis and tris imidazolium based ILs containing 1,3,5 alkylidene 2,4,6 trimethyl benzene linkers, where the alkyl arm could be a methylene fragments (Fig. 1). In this study prepared multi-cationic ILs, can be used for syntheses of a 2-amino-4H-chromenes under microwave irradiation.
Results and discussion
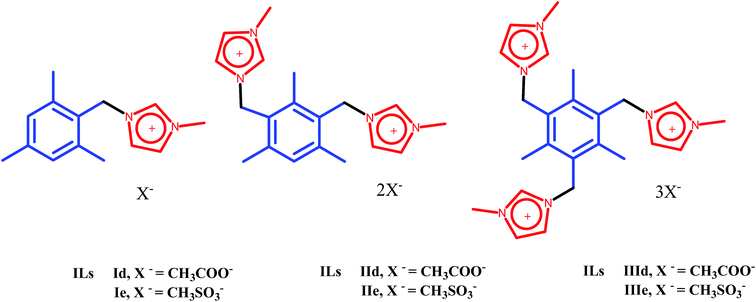
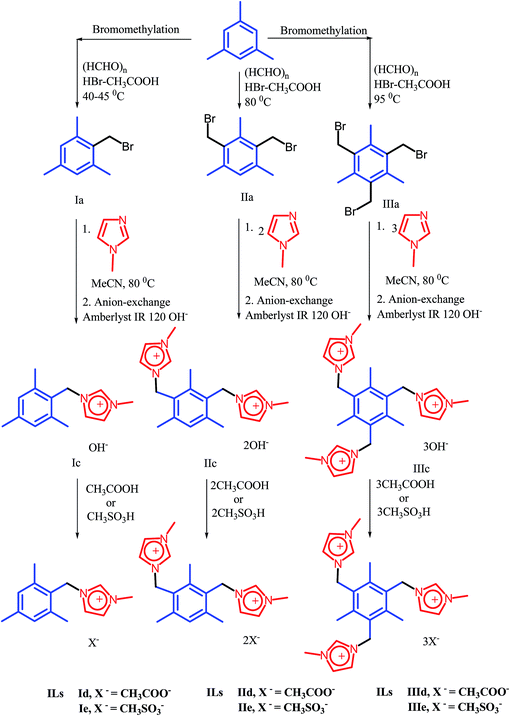
A development of a novel synthetic route to the imidazolium IL scaffold that has one, two and three imidazolium cations with mesitylene backbone and each cation entity paired with two different anions (CH3COO and CH3SO3−) is described in Scheme 1.The known compounds mono, bis and tris (bromomethyl) mesitylenes (Ia, IIa, and IIIa) were synthesized from the mesitylene according to the procedure described by Made et al.24 The imidazolium bromide salts (Ib, IIb and IIIb) were obtained in high yields (95, 95 and 96 %) by treatment of compounds Ia, IIa and IIIa with 1-methyl imidazole in acetonitrile at 80 °C. Ion-exchange approach was used for synthesis of hydroxide salts Ic, IIc and IIIc as reported by Fukumoto et al.25 The method uses the hydroxide loaded resin (Amberlyst IR 120) to displace the bromide anion from the bromide salts Ib, IIb and IIIb to produce the corresponding hydroxide salts Ic, IIc and IIIc. The absence of any bromide ions in aqueous ILs solution was confirmed by AgNO3 test. The concentration of hydroxide ions in aqueous solution of salts Ic, IIc and IIIc was determined by titration with standard HCl (0.1 N) solution conductometrically. Aqueous salt solutions of Ic, IIc and IIIc were then treated with equimolar amount of acetic acid and methane sulphonic acid at room temperature. The desired acetate ILs (Id, IId and IIId; 95, 95 and 96 % respectively) and methane sulphonate ILs (Ie, IIe and IIIe; 96, 95 and 96 % respectively) were isolated in excellent yields by simple evaporative removal of the water. The resin material was recycled using aqueous sodium hydroxide solution (1.0 M). ILs IId, IIe, IIId and IIIe are transparent and yellowish colored liquids at room temperature, while ILs, Id and Ie are solids at room temperature and melt at 90–92 °C and 87–89 °C respectively. The structures of newly synthesized ILs have been confirmed on the basis of IR, 1H and 13C NMR data.
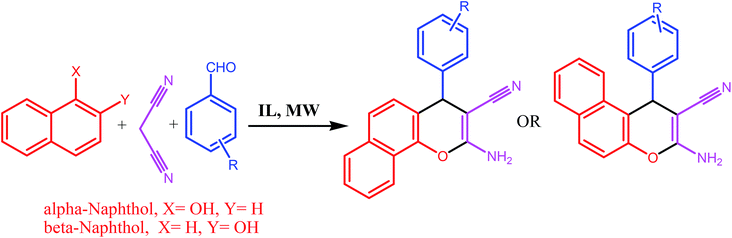
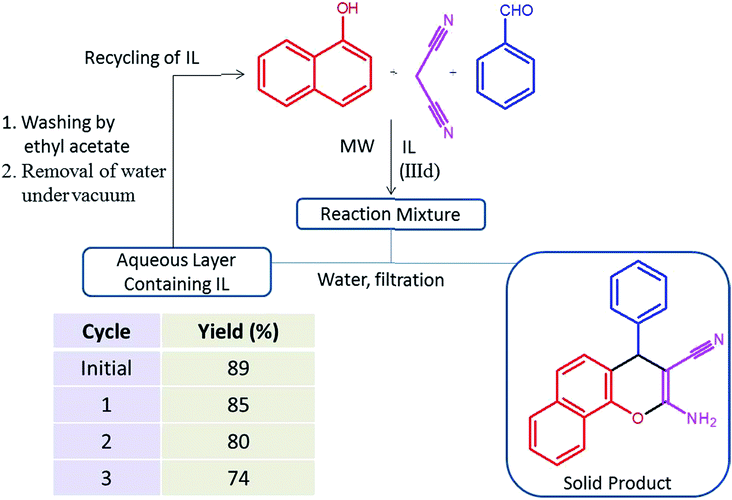
Next, these ILs were employed as a catalyst and reaction media for the synthesis of 2-amino-4H-chromene under microwave heating (100 W). An initial screening was done using benzaldehyde, malononitrile and α-naphthol as starting materials to obtain maximum yield of the product in shortest reaction time under microwave heating in IL IIId (Scheme 2). The progress of the reaction was monitored by TLC.
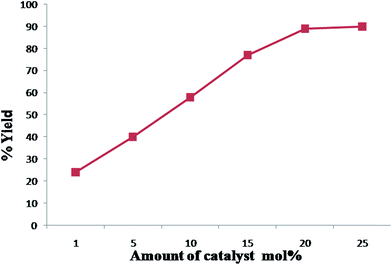
To optimize the quantity of IL required for the catalytic effect, the above model reaction was carried out in presence of 1, 5, 10, 15, 20 and 25 mol% of IL IIId (Fig. 2). Optimization of IL concentration showed that 20 or 25 mol% IL was found to be the most effective with a high yield (89%) of the desired product in 7 min. The lower IL concentrations gave less yields of the desired product.
Next, the influence of the number of imidazolium ions as well as the nature and number of counter ions on the catalytic efficiency of the ILs (20 mol%) was investigated. The results revealed in Table 1 shows that not only the number of imidazolium ions, but also the nature and number of counter anions makes a significant influence on the catalytic efficiency of the ILs. Thus, ILs Id, IId, and IIId showed high activity while ILs Ie, IIe and IIIe showed moderate catalytic activity under the identical conditions. It is worth of note that although the similar conversions were obtained for the IL Id, IId, and IIId (Table 1, entries 1, 3 and 5), the reaction in IL IIId required less time. It may be due to the presence of three acetate anions in the IL framework. To demonstrate effect of microwave heating, the model reaction was investigated in presence of IIId under conventional heating at 100 °C (Table 1, entry 7). The reaction was sluggish indicating that microwaves remarkably increases the rate of the reaction due to the synergistic couple with ILs.
| Entry | IL | Time (min) | Yieldb |
|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), malononitrile (1 mmol) and α-naphthol (1 mmol), IIId (20 mol%), under microwave irradiation.b Isolated yields after purification.c Reaction by conventional heating at 100 °C. | |||
| 1 | Id | 12 | 83 |
| 2 | Ie | 17 | 56 |
| 3 | IId | 9 | 83 |
| 4 | IIe | 16 | 62 |
| 5 | IIId | 7 | 89 |
| 6 | IIIe | 14 | 66 |
| 7c | IIId | 180 | 85 |
To establish the generality of the catalytic activity of IIId in synthesis of chromene, various aromatic aldehydes were reacted with α and β-naphthols under microwave heating. The results are depicted in Table 2. The electron poor electron rich and sterically hindered benzaldehydes the corresponding 2-amino-4H-chromenes were obtained in good to excellent yields. The electronic property of the substituent on the aromatic ring of aldehyde had a noticeable effect on the yield of product. The aryl aldehydes bearing electron withdrawing groups gave slightly better yields than those with electron donating groups.
| Entry | Aryl aldehyde | Chromene | Time (min) | Yieldb (%) | Mpc (°C) | |
|---|---|---|---|---|---|---|
| Observed | Reported | |||||
| a Reaction conditions: aldehydes (1 mmol), α/β-naphthol (1 mmol), malononitrile (1 mmol), IIId (20 mol%), under microwave heating.b Isolated yields after purification.c Identified by comparison with literature.10,12,13,15,26 | ||||||
| 1 |  |
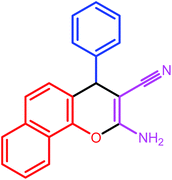 |
7 | 89 | 209–210 | 210–211 |
| 2 |  |
 |
10 | 79 | 235 | 236–237 |
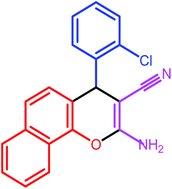
| 3 | 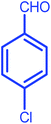 |
 |
7 | 83 | 231–232 | 232 |
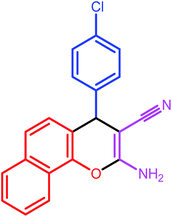
| 4 | 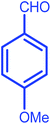 |
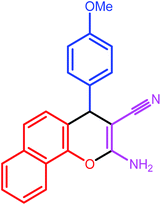 |
9 | 80 | 190–191 | 191 |
| 5 |  |
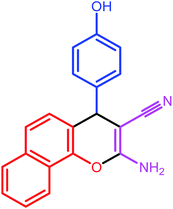 |
10 | 79 | 244–245 | 245–247 |
| 6 | 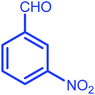 |
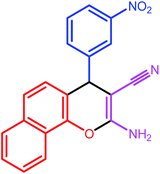 |
8 | 84 | 212–214 | 212–214 |
| 7 | 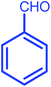 |
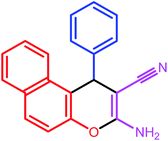 |
7 | 87 | 279–281 | 278–280 |
| 8 |  |
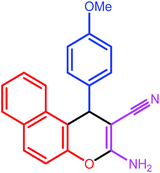 |
9 | 80 | 183 | 182 |
| 9 | 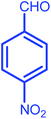 |
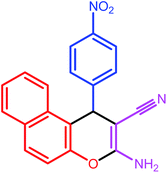 |
8 | 86 | 188–189 | 188 |
| 10 |  |
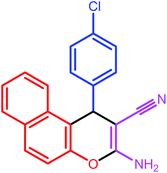 |
7 | 82 | 207–209 | 208–210 |
| 11 | 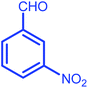 |
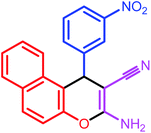 |
7 | 86 | 210–212 | 211–213 |
For any chemical process, recovery and recycling of the catalyst as well as reaction media is a very important aspect for sustainability. Compared to the traditional catalysts and solvents, easy recycling is an attractive property of many ILs. Consequently we investigated catalytic activity of recycled IL (IIId) under microwave heating using a model reaction (Fig. 3). After completion of the reaction, water (5 mL) was added in reaction mixture and the solid product was filtered off and washed with water. The recovered aqueous layer containing IL was washed with ethyl acetate (2 × 5 mL) to remove organic compounds. The aqueous layer was concentrated under vacuum and the recovered IL was reused for next reaction cycle. The recycling results showed that IL (IIId) can be recycled three times with moderate decrease in product yield.
Conclusion
In summary, a new mesitylene backbone containing acetate and methane sulphonate ILs were prepared using efficient multi-step synthetic route. In addition importantly these ILs are inherently halide-free, which offer number of benefits for the development of new IL based technologies. We have successfully combined the advantages of microwave technology with ILs to facilitate the rapid construction of chromene skeletons from readily obtainable and inexpensive materials via multi-component strategy. The operational simplicity of the procedure, shorter reaction time, simple workup, environmental friendliness, and reusability of the catalyst for a number of times without appreciable loss of activity are noteworthy features of the protocol.Acknowledgements
We gratefully acknowledge the financial support from the Science and Engineering Research Board, Department of Science and Technology (SERB-DST), Government of India, New Delhi, under the scheme of Start-Up research grants for Young Scientists (SB/FT/CS-153/2013).References
- (a) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed; (b) A. Padwa and S. K. Bur, Tetrahedron, 2007, 63, 5341–5378 CrossRef CAS PubMed; (c) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899–907 CrossRef CAS PubMed; (d) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134–7186 CrossRef CAS PubMed; (e) X.-S. Wang, J. Zhou, M. Y. Yin, K. Yang and S. Tu, J. Comb. Chem., 2010, 12, 266–269 CrossRef CAS PubMed.
- M. M. Khafagy, A. H. F. Abd El-Wahab, F. A. Eid and A. M. El-Agrody, Farmaco, 2002, 57, 715–722 CrossRef CAS PubMed.
- (a) N. R. Taylor, A. Cleasby, O. Singh, T. Skarzynski, A. J. Wonacott, P. W. Smith, S. L. Sollis, D. Howes, P. C. Cherry, R. Bethell, P. Colman and J. Varghese, J. Med. Chem., 1998, 41, 787–797 CrossRef PubMed; (b) A. Martínez-Grau and J. Marco, Bioorg. Med. Chem. Lett., 1997, 7, 3165–3170 CrossRef.
- C. P. Dell and C. W. Smith, European Patent, Appl. EP537949 Chem. Abstr. 1993119139102d.
- S. J. Mohr, M. A. Chirigos, F. S. Fuhrman and J. W. Pryor, Cancer Res., 1975, 35, 3750–3754 CAS.
- F. Eiden and F. Denk, Arch. Pharm., 1991, 324, 353–354 CrossRef CAS PubMed.
- (a) E. A. Hafez, M. H. Elnagdi, A. G. A. Elagemey and F. M. A. El-Taweel, Heterocycles, 1987, 26, 903–907 CrossRef CAS; (b) G. P. Ellis, The Chemistry of Heterocyclic Compounds Chromenes, Chromanes and Chromones, ed. WeissbergerA. and TaylorE. C, John Wiley, New York, 1977 Search PubMed.
- (a) A. G. A. Elagamey and F. M. A. A. El-Taweel, Indian J. Chem., 1990, 29B, 885–886 CAS; (b) J. Bloxham, C. P. Dell and C. W. Smith, Heterocycles, 1994, 38, 399–408 CrossRef CAS.
- G. Sabitha, M. Bhikshapathi, S. Nayak, R. Srinivas and J. S. Yadav, J. Heterocycl. Chem., 2011, 48, 267–271 CrossRef CAS.
- (a) R. Ballini, G. Bosica, M. L. Conforti, R. Maggi, A. Mazzacani, P. Righi and G. Sartori, Tetrahedron, 2001, 57, 1395–1398 CrossRef CAS; (b) T. S. Jin, J. S. Xiao, S. J. Wang and T. S. Li, Ultrason. Sonochem., 2004, 11, 393–397 CAS.
- R. Maggi, R. Ballini, G. Sartori and R. Sartorio, Tetrahedron Lett., 2004, 45, 2297–2299 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari, V. Zadsirjan, F. F. Bamoharram and O. M. Heravi, Bioorg. Med. Chem. Lett., 2007, 17, 4262–4265 CrossRef CAS PubMed.
- (a) M. Kidwai, S. Saxena, M. K. R. Khan and S. S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295–4298 CrossRef CAS PubMed; (b) Y.-M. Ren and C. Cai, Catal. Commun., 2008, 9, 1017–1020 CrossRef CAS.
- B. S. Kumar, N. Srinivasulu, R. H. Udupi, B. Rajitha, Y. T. Reddy, P. N. Reddy and P. S. Kumar, J. Heterocycl. Chem., 2006, 43, 1691–1693 CrossRef CAS.
- B. Baghernejad, M. M. Heravi and H. A. Oskooie, J. Korean Chem. Soc., 2009, 53, 631–634 CrossRef CAS.
- A. Solhy, A. Elmakssoudi, R. Tahir, M. Karkouri, M. Larzek, M. Bousmina and M. Zahouily, Green Chem., 2010, 12, 2261–2267 RSC.
- D. Kumar, V. B. Reddy, B. G. Mishra, R. K. Rana, M. N. Nadagouda and R. S. Varma, Tetrahedron, 2007, 63, 3093–3097 CrossRef CAS.
- Organic Electrochemistry, ed. H. Lund, Marcel Dekker, New York, 2000 Search PubMed.
- M. N. Elinson, A. S. Dorofeev, S. K. Feducovich, S. V. Gorbunov, R. F. Nasybullin, N. O. Stepanov and G. I. Nikishin, Tetrahedron Lett., 2006, 47, 7629–7633 CrossRef CAS.
- (a) J. Dupoint, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3692 CrossRef; (b) J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2, 2133–2140 RSC; (c) T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed; (d) M. J. Earle, P. B. McCormac and K. R. Seddon, Green Chem., 1999, 1, 23–25 RSC.
- (a) C. Oliver Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef PubMed; (b) V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629–639 CrossRef CAS PubMed.
- (a) M.-P. Rafael, J. Mex. Chem. Soc., 2007, 51, 252–264 Search PubMed; (b) R. Martínez-Palou, Mol. Diversity, 2010, 14, 3–25 CrossRef PubMed; (c) L. Buriol, C. P. Frizzo, L. D. T. Prola, D. N. Moreira, M. R. B. Marzari, E. Scapin, N. Zanatta, H. G. Bonacorso and M. A. P. Martins, Catal. Lett., 2011, 141, 1130–1135 CrossRef CAS.
- G. Rashinkar, S. Kamble, A. Kumbhar and R. Salunkhe, Transition Met. Chem., 2010, 35, 185–190 CrossRef CAS.
- A. D. van der Made and H. van der Made, J. Org. Chem., 1993, 58, 1262–1263 CrossRef CAS.
- K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2009, 127, 2398–2399 CrossRef PubMed.
- L. Chen, X. J. Huang, Y. Q. Li, M. Y. Zhou and W. J. Zheng, Monatsh. Chem., 2009, 140, 45–47 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: IR, 1H NMR and 13C NMR data of the compounds. See DOI: 10.1039/c6ra01062h |
| This journal is © The Royal Society of Chemistry 2016 |