Fluorescent gold nanoclusters for in vivo target imaging of Alzheimer's disease†
Lanmei Laia,
Chunqiu Zhaoa,
Xiaoqi Lib,
Xiaoli Liua,
Hui Jianga,
Matthias Selkec and
Xuemei Wang*a
aState Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China. E-mail: xuewang@seu.edu.cn; Tel: +86-25-83792177
bNanjing Foreign Language School, Nanjing 210096, China
cDepartment of Chemistry and Biochemistry, California State University, Los Angeles, California 90032, USA
First published on 14th March 2016
Abstract
Alzheimer's disease involves the formation of numerous senile plaques (SPs) composed of β-amyloid (Aβ) peptides. At the same time the level of various redox-active metal ions changes leading to a completely different redox environment in the brains of Alzheimer's patients compared with the normal brain. Given these considerations, we have explored a new strategy of in vivo rapid fluorescence bio-imaging of Alzheimer's disease through target bio-labeling of the diseased sites. The sites are exposed to aqueous solutions of chloroauric acid (HAuCl4), which subsequently forms Au salts. These salts then self-assemble into gold nanoclusters (Au NCs), which can then be used for fluorescence imaging. For instance, taking Alzheimer's model mice (AD) and a normal control group of mice (NOR) as models, our studies demonstrate that intravenous injection of aqueous HAuCl4 through the AD mice tails allowed accurate bright fluorescence labeling around the affected sites of the Alzheimer's brain within a couple of hours, while this did not occur in normal mice (controls): the control mice did not exhibit any fluorescent areas even 24 h after intravenous injection of an identical or larger amount of aqueous HAuCl4. These observations suggest that HAuCl4 molecules could readily pass through the blood–brain barrier (BBB) of the AD mice and then mainly accumulate on the hippocampus specific region, followed by in situ biosynthesis of the Au NCs for fluorescent labeling of the affected sites of the Alzheimer's brain. This strategy provides a new method for the rapid and early diagnosis of Alzheimer's disease and may have potential for effective theranostics of Alzheimer's disease.
1. Introduction
Alzheimer's disease involves irreversible neuronal degeneration, and is the most common cause of dementia in middle-aged and aged people.1 The medical community has been attempting to find ways to diagnose and cure the disease ever since Alzheimer's discovery of the disease in 1907. However, to date, there is still no effective method for early diagnosis and treatment of Alzheimer's disease.It is already known that Alzheimer's disease is a multi-factorial neurodegenerative disorder. Among these factors, age is arguably the most important one, followed by genetic factors and the presence of trace elements including aluminum, iron, zinc, selenium and others. In addition, brain injury, poisoning, metabolic and endocrine diseases, vitamin deficiency, and ischemia and hypoxia in the brain can also lead to Alzheimer's disease.2–5 There are numerous hypotheses regarding Alzheimer's disease's pathogenesis including formation and metabolic disorders of Aβ, abnormal Tau protein phosphorylation,6 the metal ions hypothesis, the involvement of oxidative stress, and others. Thus the specific mechanism causing the Alzheimer's disease is still not understood. Pathologically, Alzheimer's disease is characterized by three hallmark elements: numerous senile plaques (SPs) composed of β-amyloid (Aβ) peptides, abundant neurofibrillary tangles (NFTs) formed by filaments of highly phosphorylated tau proteins, and apparent loss of neurons in the brain.7–9 All these process happens primarily on the hippocampus and cerebral cortex area of the brain, which are associated with learning and memory function, and, as a result, the major symptoms patients showed clinically progressive memory loss and cognitive impairment. Alzheimer's disease has a very long incubation period. If it can be diagnosed at a very early stage, followed by treatment at the early stage, the Alzheimer's patient's quality of life and rate of survival might be significantly improved. Therefore, early diagnosis should play a vital role in treating and prevention of this disease.
At present, several imaging technologies are used in the clinical diagnostics of Alzheimer's disease, namely computerized tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). However, there still exist some disadvantages in the sensitivity and specificity,10 i.e., CT can not accurately show the hippocampal formation which is the main focal areas of Alzheimer's disease; MRI is an expensive diagnostic tool and the long scanning time is sensitive to movement of the patient's body possibly leading to artifacts in the scan. PET diagnostics involves radiation labels, including radioactive isotopes. Hence, early diagnose of Alzheimer's patients is difficult.
Gold has actually been utilized to improve the efficiency of medicines and medical devices in ancient China and Arabia. In addition to the traditional applications such as biomedical dentistry,11 gold has also been found various applications in drug delivery systems,12 for cancer sensitive optical imaging and treatment,13,14 and bio-molecular manipulation through nanotechnologies.15–17 Our recent studies have shown that bio-synthesized fluorescent gold or silver nanoclusters (NCs) can be readily utilized as a potential probe for highly sensitive optical imaging of cancer at both the cellular and animal levels.18–20
It is known that one of the main pathological characteristics of Alzheimer's disease is senile plaque formation in the brain. Its major component is Aβ peptides which play a pivotal role in the process of Alzheimer's disease.21–23 It has already been found that Aβ's oligomers such as Aβ1–40 and Aβ1–42 exhibit strong neurotoxicity.24,25 Healthy brain can also release Aβ during activation of neurons under physiological conditions, and the formation and clearance rate of Aβ is balanced in the normal brain. However, when Alzheimer's disease occurs, mutation or overexpression of amyloid precursor protein (APP) genes will appear, leading to Aβ's abnormal deposition in Alzheimer's brain.26 Many studies have shown that Aβ aggregation is induced by metal ions (i.e., Zn2+, Cu2+, Fe3+, Al3+, and others). Thus, Aβ will be continuously generated, aggregated and accumulated in Alzheimer's brain when it is combined with these metal ions, resulting in formation of a large amount of free radicals.27–30 Meanwhile, upon accumulation, Aβ may cause a series of complex reactions leading to neurotoxic products, and Aβ may damage the mitochondria and disturb the respiratory chain, which leading to additional oxidative stress via formation of superoxide anion.31 Additionally, Aβ can activate the microglia for triggering inflammatory reactions, cause neuronal cell membrane damage, and then increase the cell membrane's penetrability, followed by a large Ca2+ influx in cells, which will induce cellular apoptosis. Moreover, it can promote tau protein phosphorylation by activating protein kinases, which will aggregate to NFTs. These pathological changes will in turn lead to Aβ's formation and deposition. Thus, the imbalance of Aβ's formation and clearance is the initiation factor for neuron degeneration and dementia, which in turn will lead to a more reducing environment in Alzheimer's brain. We therefore suggest that an electron acceptor such as HAuCl4 may rapidly generate in situ fluorescent gold nanoclusters inside the AD's brain. Gold nanoclusters are composed of up to hundreds of stacked metal atoms. The actual size of the gold nanoclusters is close to the Fermi wavelength. The characteristics are similar to semiconductors which can generate a specific separation of energy level and emit fluorescence under certain excitation wavelength.
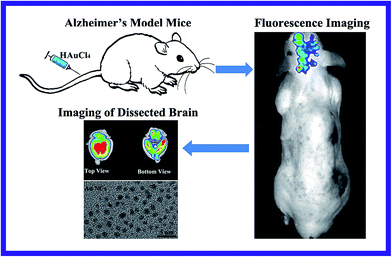
On the basis of this hypothesis, in this study we have explored a new strategy of in vivo rapid fluorescence imaging of the affected brain sites of Alzheimer's disease by precise bio-marking through in situ biosynthesized fluorescent gold nanoclusters formed from HAuCl4. Our approach is based on the fact that the redox environment in the AD brain (i.e., relatively low oxygen metabolism and more free radicals) is very different from the normal brain. The Aβ formed in AD may also accumulate in the mitochondria and affect the respiratory chain, which may cause oxidative stress possibly via superoxide anion formation from inefficient respiration.32 Meanwhile, it is known that the BBB is a system of cells that regulates the exchange of substances between the brain and the blood. There are some prerequisites for the exogenous substances passing through the BBB such as the molecules are small enough to directly pass through the BBB by the intercellular space spread. As described in Fig. 1, using Alzheimer's model mice (AD) as an experimental model, we have found that HAuCl4 molecules can readily pass through the BBB of the AD mice and may undergo a more rapid and efficient spontaneous reduction into gold nanoclusters inside the affected brain areas of the ADs compared with normal mice. The biosynthetic process appears to correlate with the high inflammatory status of the affected sites. It is known that when Alzheimer's occurs, in addition to a large amount of ascorbate, considerably elevated levels of hydrogen peroxide and other free radicals and redox ligands may also appear in lesion locations in the AD brain, which could possibly interact with HAuCl4. Our recent study also demonstrates that HAuCl4 can readily interact with GSH, and the additional thiol ligands can also efficiently attach to the surface of Au nanoparticles and rapidly induce bright photoluminescence.28 Since the hippocampus is the first and most seriously affected area in the AD brain, the biosynthesized gold nanoclusters may mainly form in the hippocampus, where the AuCl4− ions could readily accumulate in hippocampus of the ADs leading to rapid biosynthesis of the fluorescent gold nanoclusters in the affected area. This raises the possibility of providing a new way for the rapid bio-marking/bio-imaging of the affected locations of AD's brain, which could possibly be utilized in future clinical application through further combining with other imaging methods such as X-ray or CT imaging for efficient multimodal imaging of the target diseased sites.
2. Experimental
In vivo bio-imaging study
Chloroauric acid trihydrate (HAuCl4·3H2O), ascorbate and ferrous chloride used in the experiments were purchased from Sigma Company. Milli-Q water (Millipore M-Q purification system, 18.2 MΩ cm) was used in all experiments. In our previous work, toxicity of HAuCl4 solution to different kinds of cells has already been explored, and we have shown that HAuCl4 solution has very low cytotoxicity when it is at lower concentration, so it can be used for in vivo fluorescence imaging.18 Moreover, in this experiment, the pH of HAuCl4 solution was adjusted to 7, which is low stimulation to the animal. Furthermore, in the relevant in vivo study it is via tail-vein injection of HAuCl4 solution that is much safer and more feasible, rather than local injection which has relatively higher toxicity.Four-month-old APP/PS1 male mice were purchased from Beijing Xiehe animal centers and the normal control group of nude male mice (NOR) were bought from Nanjing animal centers. All animals were kept in clean facilities with a 12 hour light/dark cycle and received water and food via a semi-barrier system.
The mice were assigned to different groups randomly for experiments (i.e., 4 mice per group). The normal control group of mice, blank control group of Alzheimer's mice (CON), and Alzheimer's model mice were utilized as the experimental models, in which the CON was not given HAuCl4, and the NOR, AD group were given different concentrations of HAuCl4 solution prepared in ultrapure H2O via tail-vein injection. In the experiments, all the mice were anesthetized first and then were removed the fur of their whole bodies for better imaging by using Perkin Elmer in vivo imaging system (IVIS Lumina XRMS Series III). For bio-imaging of the relevant diseased area in the brain location, the mice were fully anesthetized by inhalation of a mixture of oxygen with 5% isoflurane, followed by fluorescence imaging at times of post-injection for 1 h (1 hour), 6 h (6 hours), 18 h (18 hours), 30 h (30 hours), etc.
The in vivo bio-imaging were acquired on a Perkin Elmer animal imaging system (IVIS Lumina XRMS Series III, with excitation wavelength of 420 nm and emission wavelength monitored at 620 nm or 670 nm). The ROI (regions of interest) analysis was conducted with the assistance of Perkin Elmer Image software. All experiments related mice were approved by the National Institute of Biological Science and Animal Care Research Advisory Committee of Southeast University, and experiments were conducted following the guidelines of the Animal Research Ethics Board of Southeast University.
Histopathologic analyses of tissues
After injection of HAuCl4 solution via tail-vein injection for 30 h, the main organs and tissues samples were harvested from model mice post-injection. Afterwards, all the mice were sacrificed. The livers, spleens, and kidneys were extracted from the control mice and Alzheimer's model mice, and then fixed in a 4% paraformaldehyde solution. The organs were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). The histological sections were observed under an optical microscope. These images were acquired by using a BX53 microscopy system (Olympus, Tokyo, Japan) that was equipped with a color CCD (DMK 41BU02, Sony Co., Tokyo, Japan).Characterization of in vivo biosynthesized gold nanoclusters
Gold nanoclusters were biosynthesized in situ in AD's brain via tail-vein injection of 1 to 10 mmol L−1 HAuCl4 solution. In order to obtain the biosynthesized gold nanoclusters, the repetitive freeze–thaw cycle method, using a similar methodology as previously reported in the literature,18 was chosen to break the AD brain tissue. Firstly, the AD brain tissue was put into −80 °C liquid nitrogen for 3 minutes, and then taken out of the liquid nitrogen and quickly put in 37 °C water for 10 minutes to make it completely melt. Subsequently, a quickly repetition of the above steps was done 3 times to reduce damage for biosynthesized gold nanoclusters. The presence of biosynthesized gold nanoclusters which were extracted from AD's brain by a repetitive freeze thaw cycles was mainly characterized by transmission electron microscope (TEM), energy-dispersive spectrometer (EDS), X-ray photoelectron spectra (XPS) and scanning electron microscopy (SEM). In contrast, no formation of gold nanoclusters was observed in the brain of normal mice injected with the same amount HAuCl4.A JEM-2100 transmission electron microscope (TEM) was used to characterize the size and size distribution of in vivo biosynthesized gold nanoclusters. Energy-dispersive spectrometer (EDS) was detected to analyze the elemental in the gold nanoclusters. The valence state of gold atoms in the in vivo biosynthesized gold nanoclusters was investigated by a PHI Quantera II X-ray photoelectron spectrometer (XPS). Gold nanoclusters which was extracted from AD's brain by a repetitive freeze thaw method was dropped on the silicon wafer and dried under vacuum, and then SEM images was taken on a field-emission scanning electron microscope (Zeiss, Ultra Plus).
Additionally, ultraviolet absorption (UV), fluorescence spectroscopy (FL), infrared spectral (IR) of diluted solution of grinded AD's brain tissue were also detected by using a UV-Vis-NIR spectrophotometer (Shimadzu, UV3600), a fluorescence spectrometer (PerkinElmer, LS-55), and a thermo fisher scientific FTIR spectrophotometer (American, Nicolet Co.), respectively.
Data analysis
Data were expressed as the means ± SD (standard deviation) from at least three independent experiments. One-tailed unpaired Student's t-test was used for significance testing, and p < 0.05 was considered significant.3. Results and discussion
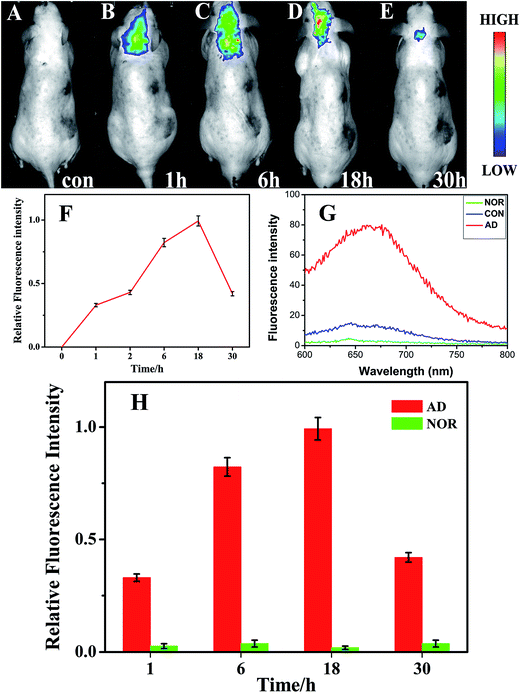
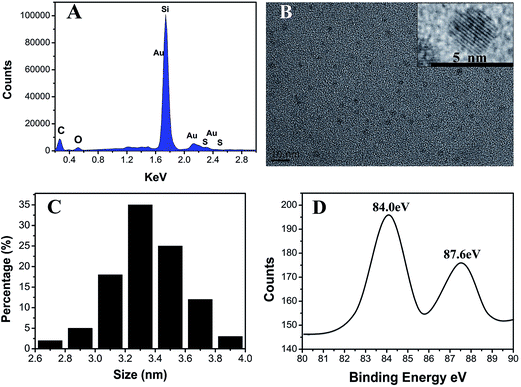
When Alzheimer's disease occurs, the redox chemistry in the brain changes. Indeed, Alzheimer's disease has several similarities to tumor which can indeed cause redox balance disorders of focus location. When Alzheimer's or tumor occurs, much more hydrogen peroxide, NADH, and free radicals may appear in lesion locations in the diseased sites of AD brain or tumors.33–36 In addition, a period of ischemia or lack of oxygen in the brain of AD may lead to a different redox environment in AD's brain compared with the normal brain. Thus, in this contribution, we have explored the possibility of the in vivo fluorescence imaging for Alzheimer's disease by bio-labeling the diseased sites via exposure to aqueous solutions of chloroauric acid. The injection of HAuCl4 leads to the self-assembly of fluorescent gold nanoclusters (Au NCs) in the affected sites. As shown in Fig. 2(A–F), upon excitation at 420 nm, fluorescent brain-bio-imaging in vivo can be achieved with an emission wavelengths maxima near 660 nm. The fluorescence spectrum of the biosynthesized Au NCs with excitation at 420 nm is shown in Fig. 2(G). The emission is spontaneously produced after tail-vein injection of HAuCl4 solution into AD's model mice, and can already be observed after one hour following injection. It is remarkable that the brain of the blank control group of mice (CON) shows almost no fluorescence after one hour. In the AD mice, the fluorescence signal increased considerably with longer circulation time after injection of HAuCl4 solution, until reaching a maximum 18 hours post-injection. Afterwards, both the fluorescence signal and the imaging area detected were found to decrease significantly, while there was almost no fluorescence in the normal control group of mice (NOR) at different time points after tail-vein injection of HAuCl4 solution (Fig. S11†). Moreover, we noted that after a relatively long time of 4–6 weeks, no changes were observed in the physical characteristics (i.e., eating, drinking, grooming, activity) and neurological status in Alzheimer's mice injected with relevant HAuCl4 solution.Interestingly, as shown in Fig. 3(A), the energy dispersive X-ray spectroscopy (EDS) observations indicate the presences of bio-synthesized gold NCs and also that no other metallic elemental impurities are present in the nanoclusters. The calculated atom content of Au reaches 0.48% (from a random region of 2 μm × 2 μm). A typical TEM image and the size distribution of the resulting Au NCs is provided in Fig. 3(B) and (C), showing that most of the Au NCs ranged between 3.1 to 3.7 nm in diameter with a distribution peak at ca. 3.3 nm. The nanoparticles are almost spherical and had no noticeable trend to aggregate. In Fig. 3(B) (inset), the HRTEM image indicates that the gold nanoclusters kept their Au–Au interplanar spacing of ∼0.2 nm. X-ray photoelectron spectroscopy (XPS) used to investigate the valence of gold atoms in the biosynthesized gold nanoclusters illustrates two peaks located at the binding energies of 84.0 and 87.6 eV, consistent with the emission of 4f photoelectrons from Au(0) (Fig. 3(D)).37
As shown in Fig. S1(A),† it is evident that no fluorescence signal can be observed in the dissected brain of the normal control mice (NOR). In contrast, bright fluorescence can readily be observed in the dissected brain of AD mice (Fig. S1(C)†). In order to explore the specific targeting ability to the various regions in the brain, the NOR and AD's brain were cut into two parts near the hippocampus area and then fluorescent imaging were obtained. As seen in Fig. S1(B and D†), a strong fluorescence signal was observed in the hippocampus compared with other areas in brain of AD mice, while no fluorescence signal was detected in the hippocampus of the NOR mice. These observations indicate that the fluorescence signal is stronger in the hippocampus, i.e. the region where the most serious brain damage in the AD occurs, exactly aligned with the pathological characteristic of Alzheimer's disease.
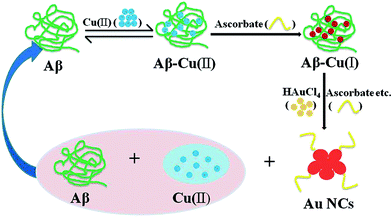
One cause of Alzheimer's disease initiation is related to high concentrations in the extracellular fluid near neurons of a cleaved protein fragment, β-amyloid, which contain a Cu(II) center with a ligation site prone to accommodate the square planar geometry Cu(II) as well as the tetrahedral one required for Cu(I).38 This allows ascorbic acid, which is present at high concentration in the brain, to reduce the Cu(II) center and initiate a catalytic reduction of dioxygen to hydrogen peroxide and peroxynitrite34 that ultimately leads to apoptosis of the surrounding neurons. Though these species cannot account for the reduction of HAuCl4 into nanoclusters (because they are oxidants), one may assume that the correspondingly high concentrations of ascorbic acid may lead to reduction of the Au(III) ions. To verify the chemical validity of this hypothesis we investigated the fate of HAuCl4 solutions submitted to ascorbic acid concentrations under conditions mimicking those found in the brain extracellular fluid. The results shown in Fig. S2, S5 and S6 in the ESI† show that gold nanoparticles did in fact form spontaneously. It is obvious that gold nanoparticles can indeed form in the presence of ascorbate, as the UV-Vis spectrum of the ascorbate–HAuCl4 reaction mixture shows a broad band centered at about 550 nm, which is assigned to the plasmon band of Au nanoparticles (2–10 nm).39,40 This provides evidence for formation of gold NPs formed in the presence of ascorbic acid and indicates that these particles are actually larger than those formed in vivo (Fig. S5–S8 in the ESI†). The larger sizes of the nanoparticles compared to those formed in ADs mice brains may simply stem from the presence of a variety of possible ligands in the brain that may stop the growth of gold nuclei at the level of nanometric clusters (Fig. 4 and S2†). Furthermore, if ascorbic acid is in fact the major reducing species in the brain, this would necessarily decrease the rate and intensity of the reduction of Cu(II) centers by ascorbic acid. Just as illustrated in Fig. 5, since there are a number of Aβ in the AD brain, Aβ can react with Cu(II) to generate Aβ–Cu(II) complex, and then Aβ–Cu(II) could be reduced into Aβ–Cu(I) leading to formation of free radicals in the presence of ascorbate in the AD brain. Meanwhile, if the AD mice was injected with HAuCl4 solution, gold nanoclusters would be readily biosynthesized in the AD brain, and at the same time, Aβ–Cu(I) would be oxidized to Aβ–Cu(II). Thus, as shown in scheme of Fig. 4, the biosynthetic processes leading to formation of Au nanoclusters could specifically occur in the affected brain of ADs. Moreover, it is already known that there exists relatively high concentration of Fe(II) in AD's brain. Thus, as observed in the in vitro experiments, Fe(II) can readily react with HAuCl4 solution at room temperature as well as in physiological temperature to generate fluorescent AuNCs, as shown in Fig. S9 and S10 in the ESI.†
On the basis of the above studies, it is evident that the bright fluorescence imaging mainly exists in the affected area near hippocampus, as shown in Fig. 2 and S1.† Moreover, the XPS-element mapping of AD's brain tissues (Fig. S2†) also confirmed the observations of relevant fluorescence imaging in the diseased area, indicating that gold nanoclusters could be specially in vivo biosynthesized and distributed in the affected area near hippocampus in AD's brain, accompanying with almost no gold nanoclusters found in other control and normal brain areas (Fig. S3 and S4†). Although presently this study is still at the stage of animal model tests, we believe that this strategy may provide a new pathway towards imaging perhaps in combination with multimode in vivo imaging strategies such as using fluorescence self-bioimaging with X-ray or CT imaging.
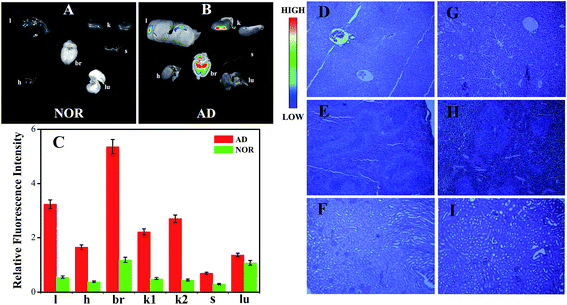
To further explore the specific targeting ability of aqueous HAuCl4 as well as the possible bio-distribution in different organs, the fluorescence imaging of major organs (i.e., liver, heart, brain, kidney, spleen and lung) of the NOR and AD mice after injected with aqueous HAuCl4 solutions have been obtained. As shown in Fig. 5(A–C) the results suggested that there is no fluorescence signal in any of the major organs of the NOR mice. While strong fluorescence signals appeared in the brain of AD mice, very weak or almost no fluorescence signals were detected in other organs of AD mice. These results demonstrate that HAuCl4 molecules can easily pass through the blood–brain barrier and then accumulate on the hippocampus specific region followed by in situ formation of the Au NCs.
As shown in Fig. 5(D–I), the tissues obtained from the harvested organs (i.e., liver, spleen, and kidney) of normal control group of mice and Alzheimer's model mice were subjected to histopathological analysis. As illustrated in these histopathologic images of normal control group of mice and Alzheimer's model mice after tail-vein injection of aqueous HAuCl4 solution, H&E-stained tissue sections showed that no histopathological abnormities or lesions were observed in the organs (i.e., liver, spleen, and kidney). Besides, no changes were observed in the physical characteristics (i.e., eating, drinking, grooming, activity, weight) and neurological status in Alzheimer's mice or normal mice injected with relevant HAuCl4 solution. These observations show that intravenous injection through the AD mice tails of aqueous HAuCl4 solutions have no side effects to the major organs with the injected dose.
4. Conclusion
In summary, our studies demonstrate the possibility of the in vivo rapid-target fluorescence imaging of Alzheimer's disease through precise bio-marking of the affected locations upon exposure to aqueous solutions of chloroauric acid (HAuCl4). Our observations illustrate that rapidly fluorescent labeling and specific brain targeting of ADs could be readily realized by the in situ biosynthesized Au NCs from aqueous HAuCl4 in the affected sites of Alzheimer's brain. It is evident that intravenous injection through the AD mice tails of aqueous HAuCl4 allows observation of fluorescence around the affected sites of Alzheimer's brain, while this did not occur as fast in normal mice (controls) such that the control mice did not exhibit any fluorescent areas even 24 h after intravenous injection of identical or larger amounts of aqueous HAuCl4. As a simple, safe and inexpensive bio-imaging strategy, this method does not have the side effect of chemosynthetic nanoparticles and thus may provide a platform for early diagnosis of Alzheimer's disease.Conflict of interest
The authors declare no competing financial interests.Acknowledgements
This work is supported by National High Technology Research & Development of China (2015AA020502), the National Natural Science Foundation of China (81325011, 21327902, and 21175020), and the Major Science & Technology Project of Suzhou (ZXY2012028). M. S. acknowledges support from the NSF-PREM program (NSF Award 1523588). Meanwhile, we greatly appreciate the kind help and advice from Professor Christian Amatore in Ecole Normale Supérieure-PSL Research University.References
- P. H. Reddy, J. Neurochem., 2006, 96, 1–13 CrossRef CAS PubMed.
- A. Rauk, Chem. Soc. Rev., 2009, 38, 2698–2715 RSC.
- P. Faller, ChemBioChem, 2009, 10, 2837–2845 CrossRef CAS PubMed.
- D. J. Selkoe, Physiol. Rev., 2001, 81, 741–766 CAS.
- S. Rukhsana, M. Patrizia, M. Francesca, C. Roberta, B. Mauro and B. D Allan, J. Alzheimer's Dis., 2010, 24, 77–84 Search PubMed.
- P. Lewczuk, H. Esselmann, M. Bibl, G. Beck, J. M. Maler, M. Otto, J. Kornhuber and J. Wiltfang, J. Mol. Neurosci., 2004, 23, 115–122 CrossRef CAS PubMed.
- J. Hardy and D. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS PubMed.
- C. A. Mathis and Y. W. Wang, Curr. Pharm. Des., 2004, 10, 1469–1492 CrossRef CAS PubMed.
- P. Ashim, N. Krishna Chaitanya, M. Tanmay, T. Kishore and M. Bhubaneswar, Chem. Commun., 2015, 51, 2245–2248 RSC.
- J. L. Cummings, N. Engl. J. Med., 2004, 351, 56–67 CrossRef CAS PubMed.
- Y. Jung, J. Ban, M. Kim, K. Lee, M. Vang and H. Yong, Sci. Adv. Mater., 2014, 6, 2227–2232 CrossRef CAS.
- R. Gui, A. Wan, X. Liu and H. Jin, Chem. Commun., 2014, 50, 1546–1548 RSC.
- C. Wang, J. Li, C. Amatore, Y. Chen, H. Jiang and X. Wang, Angew. Chem., Int. Ed., 2011, 50, 11644–11648 CrossRef CAS PubMed.
- M. H. Oh, J. H. Yu, I. Kim and Y. S. Nam, ACS Appl. Mater. Interfaces, 2015, 7, 22578–22586 CAS.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS PubMed.
- K. Sato, K. Hosokawa and M. Maeda, J. Am. Chem. Soc., 2003, 125, 8102–8103 CrossRef CAS PubMed.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed.
- J. Wang, G. Zhang, Q. Li, H. Jiang, C. Liu, C. Amatore and X. Wang, Sci. Rep., 2013, 3, 1157 Search PubMed.
- S. Gao, D. Chen, Q. Li, J. Ye, H. Jiang, C. Amatore and X. Wang, Sci. Rep., 2014, 4, 4384 Search PubMed.
- J. Wang, J. Ye, H. Jiang, S. Gao, W. Ge, Y. Chen, C. Liu, C. Amatore and X. Wang, RSC Adv., 2014, 4, 37790–37795 RSC.
- L. Xiao, L. Wei, L. Ying, W. Dan, Z. Cui, H. Zhong and T. Xiang, Chem. Commun., 2013, 49, 5865–5867 RSC.
- T. Amantha and D. S. Bart, Sci. Signaling, 2009, 2, re8 Search PubMed.
- J. I. Kourie, Cell. Mol. Neurobiol., 2001, 21, 173–213 CrossRef CAS PubMed.
- J. A. Hardy and G. A. Higgins, Science, 1992, 256, 184–185 CAS.
- T. Kai, L. Zhang, X. Wang, A. Jing, B. Zhao, X. Yu, J. Zheng and F. Zhou, ACS Chem. Neurosci., 2015, 6, 879–888 CrossRef CAS PubMed.
- M. S. Wolfe, Biol. Chem., 2012, 393, 899–905 CrossRef CAS PubMed.
- P. G. K. Sengupta, B. Sahoo, S. Yuan, D. J. E. Callaway and S. Maiti, Biochemistry, 2003, 42, 10506–10513 CrossRef CAS PubMed.
- X. Su, H. Jiang and X. Wang, Anal. Chem., 2015, 87, 10230–10236 CrossRef CAS PubMed.
- O. Wirths, G. Multhaup and T. A. Bayer, J. Neurochem., 2004, 91, 513–520 CrossRef CAS PubMed.
- M. Cuajungco, L. A. Goldstein, M. Smith, J. Lim, C. Atwood, X. Huang, Y. Farrag, G. Perry and A. Bush, J. Biol. Chem., 2000, 275, 19439–19442 CrossRef CAS PubMed.
- R. M. Koffie, T. Hashimoto, H. C. Tai, K. R. Kay, A. Serrano-Pozo and D. Joyner, Brain, 2012, 135, 2155–2168 CrossRef PubMed.
- P. H. Reddy and M. F. Beal, Trends Mol. Med., 2008, 14, 45–53 CrossRef CAS PubMed.
- C. Amatore, S. Arbault, M. Guile and L. Frederic, Chem. Rev., 2008, 108, 2585–2621 CrossRef CAS PubMed.
- H. Wiseman and B. Halliwell, Biochem. J., 1996, 313, 17–29 CrossRef CAS PubMed.
- D. Trachootham, J. Alexandre and P. Huang, Nat. Rev. Drug Discovery, 2009, 8, 579–591 CrossRef CAS PubMed.
- G. P. Bienert, J. K. Schjoerring and T. P. Jahn, Biochim. Biophys. Acta, 2006, 1758, 994–1003 CrossRef CAS PubMed.
- T. F. Jaramillo, S. H. Baeck, B. R. Cuenya and E. W. McFarland, J. Am. Chem. Soc., 2003, 125, 7148–7149 CrossRef CAS PubMed.
- R. Giacovazzi, I. Ciofini, L. Rao, C. Amatore and C. Adamo, Phys. Chem. Chem. Phys., 2014, 16, 10169–10174 RSC.
- A. B. R. Mayer and J. E. Mark, Eur. Polym. J., 1998, 34, 103–108 CrossRef CAS.
- J. C. G. Martínez and R. M. Crooks, J. Am. Chem. Soc., 2004, 126, 16170–16178 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fluorescence imaging of the dissected brain of the normal control group of mice and Alzheimer's model mice, XPS-element mapping of AD's and NOR's brain tissues, ultraviolet absorption spectra of ascorbate and CuSO4 solution alone as well as the mixture of CuSO4 and ascorbate solution upon addition of HAuCl4 solution at different time points, typical TEM image, HRTEM image and the size distribution of the resulting Au NCs separately from the in vitro reaction of the mixture of ascorbate solution and HAuCl4 solution, the mixture of FeCl2 solution and HAuCl4 solution, and fluorescence imaging of the normal control group of mice at different time points via tail-vein injection of HAuCl4 solution. See DOI: 10.1039/c6ra01027j |
| This journal is © The Royal Society of Chemistry 2016 |