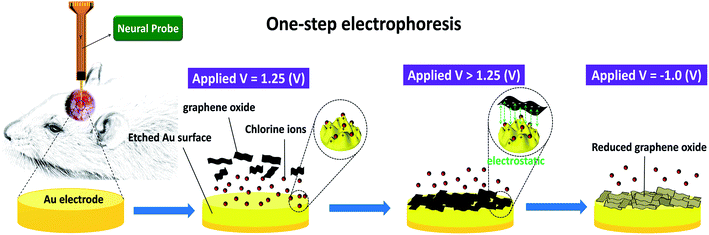
Newly reduced graphene oxide/gold oxide neural-chemical interface on multi-channel neural probes to enhance the electrochemical properties for biosensors
Ta-Chung Liua,
Chao-Yi Chua,
You-Yin Chenb and
San-Yuan Chen*a
aDepartment of Materials Science and Engineering, National Chiao Tung University, No. 1001, Ta-Hsueh Rd., Hsinchu, Taiwan 300, Republic of China. E-mail: sanyuanchen@mail.nctu.edu.tw
bDepartment of Biomedical Engineering, National Yang Ming University, No.155, Sec. 2, Linong St., Taipei, Taiwan 112, Republic of China
First published on 8th March 2016
Abstract
In this study, a facile one-step Cyclic Voltammetry (CV) electrophoresis was proposed for designing a reduced graphene oxide/gold oxide (rGO/AuOx) modified electrode by using chloride ions (Cl−) with the simultaneous occurrence of gold oxidation and GO reduction to induce the intimate attachment of negatively charged rGO sheets on the positively charged Au+/Au3+ clusters by electrostatic interaction. The surface microstructure and the oxygen functional groups of rGO/AuOx can be tuned by controlling the dissolution rate of gold via the deposition scan rate. At a low deposition scan rate, the rGO/AuOx electrode with well-dispersive rGO sheets and large active sites can induce rapid electron transfer to promote H2O2 detection. The amperometric response results displayed a relative fast response of less than 5 s with a low detection limit of 0.63 μM (S/N = 3). Also, the rGO/AuOx neural-chemical interface can be modified at the multi-channels on neural probe and they exhibited excellent sensing performance to H2O2. The results demonstrated that the rGO/AuOx modified electrode integrated with a neural probe using this one-step electrochemical deposition can provide a faster response and higher sensitivity by optimizing and controlling the surface microstructures of rGO/AuOx, which would serve as a platform for medical application such as biosensors for multi-sensing.
1. Introduction
Over the past decade the development of non-enzymatic sensors has gained wide attention because the performances of enzyme-based assays are easily affected by the environmental conditions such as temperature, pH, humidity, and the presence of enzyme-poisoning molecules and the enzymatic sensors are easily dampened by an inflammatory response during implantation.1–3 The fabrication of non-enzymatic biosensors with inorganic metal oxides, polymers, carbon materials, nanoparticles, and their composites have introduced good selectivity and high responsiveness so that non-enzymatic sensors have become primary option for in vivo sensing. Recently, the application of nanomaterials has gained considerable attention to address non-enzymatic sensor challenges by increasing geometry areas and chemical activity to enhance sensitivity and selectivity. These challenges directed us toward developing a nanocomposite modified electrode with improved electrochemical/electrical performances and sensitivity, particularly for micro-scale electrochemical sensors because of several advantages for the detection of biochemical signals compared to macroscopic counterparts: (1) higher spatial resolution because of small geometric area (i.e. selectivity)4 (2) small RC (R: resistance, C: capacitance) time constants due to the reduced double layer capacitance, providing higher temporal resolution and faster electron transfer.5,6The performance of an electrode depends on the materials used and the ability to chemically modify its surface, which can be further tuned by controlling the architecture of the electrode interface using nanomaterials. Among different nanomaterials, graphene-based have drawn more attention due to its unique properties and highly amenable to micro-fabrication. Moreover, graphene oxide-based (GO-based) materials are electrochemically active toward redox-active species and achieve intimate connectivity with molecules on their surface due to their excellent electrical conductivity, mechanical properties, chemical stability, and biocompatibility.7 Moreover, owing to their solution-processability,8 GO-based materials can be readily “functionalized” to enhance catalytic properties9–14 and reduce thermal noise in recording signals.15 However, certain problems such as aggregation or restacking were encountered in assembling a GO-modified metal-based sensor due to the very weak intersheet van der Waals attractions and non-comparable bonding between metal and GO sheets. Many methods such as Langmuir–Blodgett deposition16,17 have been employed to synthesize single or monolayer GO to resolve restacking issues and enable the film thickness to be controlled whereas the disadvantages of weak physical bonding by intermolecular forces and the addition of toxic materials during the manufacturing process must still be overcome. Other methods for overcoming the problems associated with GO sheet restacking have involved the intercalation of nanoparticles such as polymer particles and even carbon nanotubes in between GO sheets.18,19 For instance, graphene nanosheets and chitosan are frequently used together because the positively charged chitosan can interact with the negatively charged graphene nanosheets to prevent their aggregation.20 However, these synthesis processes are highly complicated because of the complex chemical reactions involved.
To solve the existing challenges and problems concerning GO-based deposition on metal electrodes, we proposed a novel facile process combined Cyclic Voltammetry (CV) with repetitive cycles and chloride ion (Cl−) induction as shown in Scheme 1. More importantly, the microstructure of the rGO/AuOx electrode can be altered by tuning the deposition scan rate so that controllable electrode-interface morphology can be obtained, which would further modify its defect density and oxygen functional groups. Hydrogen peroxide (H2O2) is closely related to human metabolism and the accumulation of H2O2 can cause grievous injury to cells through base modifications and strand breakage in genomic DNA. Owing to the importance of H2O2, the sensitive and precise determination of H2O2 is mandatory and highly appreciable. By using this unique one-step electrophoresis in this work, we made a proof-to-concept on multi-sensing by evaluating the sensing performance of H2O2 with the rGO/AuOx nanocomposite electrodeposited on multi-channel neural probe. The results have demonstrated that the rGO/AuOx nanocomposite modified electrode with tunable microstructures exhibited excellent sensitivity for H2O2 detection, implying the potential application in biosensors.
2. Materials and methods
2.1. Preparation of graphene oxides
The small-sized GO sheets providing large amount of active sites for micro-biosensor were prepared using the modified Hummers method21 from flake graphite (no. 332461, Aldrich, USA). Briefly, 10 g of graphite and 7.5 g of NaNO3 were placed in a flask. Then, 750 mL of H2SO4 was added with stirring in an ice-water bath, and 45 g of KMnO4 was slowly added over approximately 1 h. Stirring was continued for 2 h in the ice-water bath and remained stirring for 5 days at room temperature to obtain homogeneous solution. The homogeneous solution was maintained with stirring over approximately 1 h, at 98 °C. The resultant mixture was further stirred for 2 h at 98 °C. Next, as the temperature was reduced to 60 °C, 30 mL of H2O2 (30 wt% aqueous solution) was added, and the mixture was continuously stirred for 2 h at room temperature. To remove the ions of the oxidant and other inorganic impurities, the resultant mixture was purified by repeating the following procedure cycle 15 times: centrifugation, removal of the supernatant liquid, addition of 2 L of a mixed aqueous solution of 3 wt% H2SO4/0.5 wt% H2O2 to the bottom solid, and dispersing the solid using vigorous stirring and bath ultrasonication for 30 min at a power of 140 W. Then, a similar procedure was repeated three times using 3 wt% HCl aqueous solution (2 L) and one time using H2O (2 L). Air-dried process was used to remove to obtain the desired GO sheets. The as-synthesized graphene oxide solution was subsequently purified by dialysis until its pH value reached 6.5, then was subjected to ultra-sonication for 1 h.2.2. Preparation of rGO/AuOx modified electrode
Electrochemical synthesis was performed by CV using an electrochemical instrument (CHI 614C, CH Instruments, Inc., Austin, USA) with a three-electrode configuration, in which Ag/AgCl served as the reference electrode (no. 002243, ALS Co., Ltd, Tokyo, Japan), a platinum mesh served as the counter electrode (no. 002222, ALS Co., Ltd, Tokyo, Japan) and a neural probe22 served as the working electrode. The gold electrode was in turn polished using 0.3 μm and 0.05 μm alumina powders. The GO solution was ultra-sonicated, and nitrogen was bubbled to degas the medium. A neutral aqueous GO suspension (0.5 mg mL−1, pH = 6.5) was mixed with the sodium chloride (NaCl) solution (100 mM) as the electrophoresis medium, in which NaCl was used as the electrolyte and an etching agent for the gold electrode. The rGO/AuOx nanocomposite was prepared using a scan range of −1.4 V to +1.4 V, within which the gold surface was dissolved at 1.25 V and electro-reduction of GO occurred at −1.0 V.23 Additionally, we prepared rGO/AuOx modified electrodes with different deposition scan rates from 10 mV s−1 to 250 mV s−1 for further investigation.2.3. Characterization of rGO/AuOx nanocomposite
The surface morphology of the rGO/AuOx nanocomposite was examined using scanning electron microscopy (SEM, JSM S6700, JEOL Ltd., Akishima-shi, Japan). The surface carbon structure of the nanocomposite was characterized using a DeltaNu® Raman spectrometer (Advantage 200A, SciAps, Inc., Woburn, USA). In addition, X-ray photoelectron spectroscopy (XPS, VG Escalab 250 iXL ESCA, VG Scientific, UK) was used to determine the elemental composition and chemical bonding of the rGO/AuOx nanocomposite.2.4. Electrochemical characterizations
The surface kinetics and surface reactions of rGO/AuOx electrodes were examined at various scan rates in PBS (10, 20, 40, 80, 160, and 320 mV s−1). The electrochemical performance of the rGO/AuOx electrodes with different deposition scan rates (10 mV s−1, 50 mV s−1, and 250 mV s−1) was tested using CV in a 0.2 mM hydroquinone (HQ)/0.1 M phosphate buffered saline (PBS) solution, in which a platinum plate was used as the counter electrode and Ag/AgCl was used as the reference electrode.2.5. Amperometric experiments of H2O2
In vitro amperometric experiments were carried out using a conventional three-electrode system in artificial cerebral spinal fluids (aCSF, 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 25 mM NaHCO3, 1.25 mM NaH2PO4, 20 mM glucose, 5% CO2, pH = 7.4), in which a neural probe incorporated with rGO/AuOx nanocomposite with 10 mV s−1 (Channel 1–3 as defined in Fig. 3) and non-coated electrode were used as the working electrodes. The buffer was purged with high-purity nitrogen for at least 30 min prior to each amperometric experiment aiming to protect the solution from oxygen. The amperometric measurements were carried out under stirring with the addition of H2O2. The successive injections of H2O2 concentration were 10 μM, 20 μM, and 50 μM for electrode Channel 1 to Channel 3. The calibration plot was performed by amperometric test with detection ranging from 10 μM to 300 μM.3. Results and discussion
3.1. Electrochemical deposition of rGO/AuOx nanocomposite
Fig. 1 shows the formation steps and the corresponding CV plot of a rGO/AuOx nanocomposite in the range from −1.4 V to 1.4 V at 10 mV s−1 with 10 electrodeposition cycles. During the first anodic potential scan, a large anodic peak appeared at +1.25 V.24 Step a represented the dissolution of the gold electrode by chloride ions (Cl−) in a GO/NaCl bath to form a positively charged surface, called the Au+/Au3+ surface. Step b illustrated the electrochemical deposition of GO sheets on Au+/Au3+ rough surface by electrostatic forces, and then the oxygen dissociated in water would be anchored in Au+/Au3+ surface to form a hybrid GO/AuOx nanocomposite. Step c, a small cathodic hump appeared at +0.6 V, indicating the hydration of Au surface and the analogous formation of Au(H2O)m+ or Au(H2O)m3+. This provides further evidence for the Au+/Au3+ ions formation in the solution during the electrochemical deposition of AuOx on Au electrode with modifying by GO. Step d presented the electro-reduction of GO, leading to the formation of a rougher rGO/AuOx nanocomposite. As it is well known that GO sheets are mostly decorated with epoxy and hydroxyl groups on the basal plane, while carbonyl and carboxyl groups are located at the edges. During the electrochemical deposition process, the GO sheets with negatively charged oxygen functionalities would be attracted towards the positively charged Au+/Au3+ ions from the bath solution by electrostatic driving force, and thus got anchored at the Au+/Au3+ surface. Meanwhile, the oxygen dissociated from the water should be captured immediately by the anchored Au3+ ions, resulting in the formation of rGO/AuOx composite. | ||
| Fig. 1 Real-time CV plot for the electrodeposition formation of the rGO/AuOx nanocomposite with 10 cycles was indicated with the letters corresponding to the manuscript. | ||
3.2. Morphology and structure characterization of rGO/AuOx nanocomposite
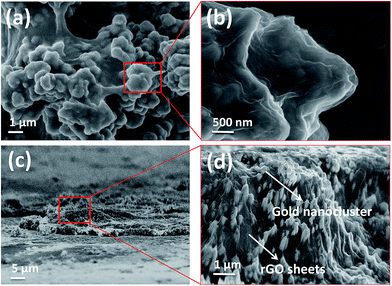
Fig. 2(a) shows the top-view surface microstructure of an rGO/AuOx nanocomposite, indicating that a blanket of wrinkled rGO sheets covered on the surface of the AuOx as presented in a magnified image of Fig. 2(b). The cross-section views with its magnified image in Fig. 2(c)–(e) showed that the flexible rGO sheets were observed to be tightly attached to the etched gold substrate (AuOx layer), revealing that the surface morphology of the rGO/AuOx nanostructure might be controlled by the AuOx layer. Such a nanostructure with a few layers of rGO sheets provided a large number of active surface sites and a rapid mass transport rate due to the short diffusion distance from rGO to AuOx. To conclude, the unique rGO/AuOx nanocomposite exhibited a high surface area, a short diffusion distance across rGO sheets to AuOx layer and the ability to tailor the surface morphology for improving the restacking of GO sheets on the electrode. Furthermore, we examine detailed morphological information of rGO/AuOx nanocomposites by AFM as shown in Fig. 3, showing that lots of sharp and smaller size protrusions, produced by chloride ions, were observed in rGO/AuOx nanocomposites deposited with 10 mV s−1, while the rGO/Au2O3 nanocomposites with 250 mV s−1 produced flattened and large bulks morphology. Furthermore, the increasing percentage of surface area and roughness (Ra) in rGO/AuOx nanocomposites with 10 mV s−1, 50 mV s−1 and 250 mV s−1 were estimated as 11.00%, 6.85%, and 3.95% and 85.8, 71.6, and 53.4 nm, respectively. These quantitative results demonstrated that the increase in surface area for the rGO/AuOx nanocomposites with lower deposition scan rates had great influence on electrocatalytic activity of the rGO/AuOx nanocomposite. Fig. 4(a) and (c) shows the Raman spectra of rGO/AuOx nanocomposites fabricated over 10, 50, and 250 mV s−1 deposition scan rates. All spectra exhibited two prominent peaks at 1350 cm−1 and 1583 cm−1, corresponding to the well-documented D and G bands, respectively. The ID/IG ratio can be used to determine the degree of bond disorder and chain information concerning carbon materials.25 As compared to GO, rGO exhibited a larger ID/IG ratio, which indicated the restoration of sp2 carbon and a decrease in the average size of the sp2 domains upon the reduction of GO. The ID/IG ratio of the rGO/AuOx nanocomposite (ID/IG = 1.245) fabricated over 10 mV s−1 was larger than that over 50 and 250 mV s−1 (ID/IG = 1.01 and ID/IG = 0.938, respectively), indicating that the amount of oxygen functional groups on the rGO/AuOx nanocomposite may be controlled by tuning the deposition scan rates and more carboxyl functional groups on the GO plane were partially electro-reduced at 10 mV s−1. As a result, a high electrical conductivity was obtained for the rGO/AuOx nanocomposites formed over low deposition scan rates XPS was further used to characterize the rGO/AuOx nanocomposite as illustrated in Fig. 4(d) and (e). The high-resolution XPS of the C1s region in Fig. 4(d) reveals that Au and O bonded with carbon in three different electronic states: carbon with a binding energy (BE) centered at 290.0 eV, carboxyl groups with a BE at 292.0 eV, and carbonates with a BE at 298.4 eV. The XPS spectrum of the O1s core level is presented in Fig. 4(e) which indicates that both C and Au bonded with oxygen as evidenced by the two peaks of 537.4 eV (Au–O) and 541.6 (C–O). The high peak intensity of Au–O indicated that the oxygen functionalities on the rGO layer might have been obscured by the strong absorption of the AuOx layer. The XPS results further proved that the etched gold electrode has been transformed into gold oxide, whereas no chemical bonding (i.e., only electrostatic forces) existed between rGO and AuOx.3.3. Interface properties tuning by deposition scan rates
The electron transfer kinetics are dependent on not only the density of electronic states but also the surface microstructure especially for the carbon basal planes with the edge plane defects,26 which would affect the electrochemical/electrical properties and sensitivity of rGO/AuOx. The surface morphology could be tailored by the ion (Cl−)-induced effect to control the dissolution rate of gold, which was also strongly dependent on the deposition scan rate associated with rGO sheets due to strong electrostatic forces between rGO and AuOx. Therefore, at a low deposition scan rate, a longer dissolution time enabled a larger coverage and high roughness structure to be generated, which promoted higher attachment amounts of rGO sheets on the AuOx layer. In contrast, when the electrochemical deposition occurred at a higher deposition scan rate, it would lead to lower coverage of the rGO/AuOx. The kinetics of the electrode interface reaction can be illustrated by the CV plot of the AuOx films obtained in 0.2 mM HQ at various scan rates as shown in Fig. 5(a). Note that the anodic and cathodic peak currents increased with the scan rate but the peak voltage (Vp) remained at the same position (58 mV) even though the scan rate varied. The results were referred to the rapid interface electron-transferring kinetics because the electron transfer was fast enough to maintain the equilibrium between the reduced and oxidized forms of the redox couple. By the Nernst equation, Fig. 5(b) presented a plot of the oxidative peak currents (Ip) as a function of the square root of the scan rate for both the anodic and cathodic peaks. The result demonstrated that both the reductive and oxidative peak currents exhibited linearity with the square root of the scan rate over the range of 10–320 mV s−1, suggesting that the redox process on the modified rGO/AuOx electrodes was predominantly diffusion-controlled mass transfer reactions.The electrochemical properties of an modified rGO/AuOx determined by the electron transfer rate were usually reflected in ΔEp, which was strongly related to the coverage of electrode materials, stacking manners vertically or laterally across the electrode surface, and diffusion distance within materials or across the interface between different materials at a heterogeneous surface. The results in Fig. 5(c) demonstrated that the redox peak current of the rGO/AuOx nanocomposite obviously increased with lowering deposition scan rates. The rGO/AuOx nanocomposite deposited at a low deposition scan rate (10 mV s−1) with 10 cycles exhibited a narrowed ΔEp (58 mV) and high sensitivity to HQ (a high peak current of ∼80 μA), approximately 3 times and 8 times than the bare gold, respectively. This was attributed to the shortened diffusion distance from rGO to the AuOx layer due to the tight connections between rGO and AuOx.
To understand the thickness effect of the rGO/AuOx nanocomposites, multiple-cycle deposition with a scan rate of 10 mV s−1 was performed, as illustrated in Fig. 5(d). The value of ΔEp decreased with increasing cycle number below 25 cycles, indicating that enhanced electron transfer kinetics occurred because the coverage of the rGO/AuOx nanocomposite increased with cycle number on the electrode. However, the increase in ΔEp with cycle number above 25 cycles was ascribed to the increased thickness of rGO on the AuOx layer, thus resulting in difficulties in transferring the electroactive species to the electrode due to the extended distance from the molecules to the electrode surface. The appearance of the minimum ΔEp value at 25 cycles (31 mV) revealed that the electron transfer kinetics were strongly correlated with the coverage and thickness of rGO on the AuOx layer, which could be easily controlled by adjusting the appropriate electrochemical parameters, a distinct advantage over previously developed methods.
3.4. Selectivity and anti-interference of rGO/AuOx electrode
The selectivity and anti-interference capability of the rGO/AuOx nanocomposite were shown in Fig. 6. The effect of common interfering electroactive substances, including dopamine (DA, 50 μM), uric acid (UA, 50 μM), glucose (50 μM) and H2O2 (50 μM) in aCSF on cyclic voltammetry was assessed and presented in Fig. 6(a). The reductive peaks were observed around −0.32 V, −0.25 V, −0.21 V and −0.5 V for UA, glucose, DA and H2O2 in aCSF, respectively. Based on the above interference study, −0.5 V was reasonably chosen as the operation voltage to enhance selectivity against the main endogenous brain interference species in an amperometric response because the applied voltage of −0.5 V in this study can distinguish the reduction from those of other electroactive substances. In the anti-interference study shown as Fig. 6(b), an obvious amperometric response (approximately 0.2 nA) appeared when 20 μM H2O2 was injected at first at an operation voltage of −0.5 V, and then glucose (20 μM) was injected in the mixture solution. When 20 μM H2O2 was added for the second time, the current changed proportionally (approximately 0.2 nA) even with the existence of the interferents. Later, although AA (20 μM), UA (20 μM) and DA (20 μM) injections as interferences produced noises, they did not cause observable amperometric changes. This demonstrated that the developed rGO/AuOx electrode showed a superior selectivity to H2O2 by applying specific voltages at −0.5 V.3.5. H2O2 sensing performance of rGO/AuOx
Overall, the rGO/AuOx nanocomposites developed in this study exhibited high charge transferring rate ensured by the exceptionally high conductivity of graphene and nanostructured conductive pathways of gold oxide (AuOx). As a result, the reduction/oxidation of H2O2 can be achieved on the rGO/AuOx nanocomposites by operating at a low voltage. In this study, the fabricated rGO/AuOx modified electrode at a deposition scan rate of 10 mV s−1 with 25 cycles was verified to operate as non-enzymatic biosensors. Fig. 7 showed the real-time amperometric i–t detection of rGO/AuOx modified electrodes in multichannel neural probe (Channel 1–3 as defined in Fig. 7(a) fabricated at 10 mV s−1, where the electrode potential was performed at −0.5 V with N2 saturated in aCSF at pH 7.4). The rGO/AuOx modified electrode (Channel 1–3) responded to amperometric currents at injections concentration of H2O2 ranging from 10 μM to 50 μM. All rGO/AuOx modified electrode obviously showed a clear stepwise, indicating that rapid oxidation/reduction reaction occurred at a very short response time, exhibiting fast electron kinetics. The current underwent a transient increasing phase in each injection step of adding H2O2 and then reached a dynamic equilibrium: this equilibrium current of all three rGO/AuOx modified electrodes (Channel 1–3) was observed to increase linearly with injected H2O2 concentrations as shown in Fig. 7(b). For the rGO/AuOx deposited with 10 mV s−1, the detection limit was 0.63 μM (signal to noise = 3) and a response time less than 5 s to reach 100% signals. The calibration plot was further indicative of the sensitivity of the rGO/Au2O3 electrode at 1024.8 nA μM−1 cm−2 (r2 = 0.997). The H2O2 sensitivity in the rGO/AuOx electrode was closely correlated with oxygen functional groups, surface roughness of the modified electrodes and the inter-attachment between rGO and AuOx. To further consider the reuse stability, the rGO/AuOx electrode was determined over a period of 14 days with analysis carried out every day shown in Fig. 8. The rGO/AuOx electrode was performed 5 times a day and the electrode was stored in 0.01 M PBS at 4 °C after test every day. The rGO/AuOx electrode was found to retain 96.1% (N = 5) of its initial activity after 14 days. Compared to other rGO/metallic oxide-based H2O2 sensors, our probe sensor with rGO/AuOx electrode displays a lower detection limit as listed in Table 1, which is probably relate to the fact that the rGO/AuOx nanocomposite deposited with 10 mV s−1 could effectively promote a two-electron coupled with two-proton redox reaction between the H2O2 and the electrode due to its extraordinary electron transfer. To sum up, the rGO/AuOx neural-chemical interface on implantable multi-channel neural probe designed with this one-step electrodeposition exhibited great tunable characteristics including electrochemical properties, low electrical impedance and high catalytic ability by varying deposition scan rates, which showed the great potential in enzymatic or non-enzymatic biosensors for in vivo neuronal chemical “multi-sensing” in brain. | ||
| Fig. 8 The reuse stability test of the rGO/AuOx electrode by measuring sensitivity over 14 days (N = 5). | ||
| Sensors | Applied potential (V) | Response time (s) | Detection limit (μM) | Reference |
|---|---|---|---|---|
| a AuNPs: gold nanoparticles; MWCNTs: multiwall carbon nanotubes; MS: microspheres. | ||||
| rGO/AuOx | −0.5 | <5 | 0.63 | This work |
| rGO/AuNPs | −0.294 | — | 6.2 | 14 |
| Cu2O–rGO | — | — | 21.7 | 27 |
| Cu2OMS–rGO | −0.24 | — | 10.8 | 12 |
| Cu2O nanocube–rGO | −0.4 | <7 | 20.8 | 28 |
| ZnO–rGO | −0.38 | <5 | 0.02 | 29 |
4. Conclusions
We have synthesized an rGO/AuOx nanocomposite using a facile one-step electrochemical method through an ion (Cl−)-induced process to tune the electrode interface with close electrostatic interaction between the negatively charged GO sheets and positively charged Au+/Au3+ substrate. The rGO/AuOx nanocomposite with low deposition scan rate provides the intact fast electron transportation from rGO to induce electron transferring on the electrode and higher H2O2 sensitivity. Most importantly, this study suggests that the tunable microstructure of rGO/AuOx nanocomposite could provide an easy way in modifying micro-biosensors and bioelectronics on multi-sensing with rapid response, high sensitivity, and well affinity.References
- M. Martín, P. Salazar, R. Villalonga, S. Campuzano, J. M. Pingarrón and J. S. González-Mora, J. Mater. Chem. B, 2014, 2, 739–746 RSC.
- P. Salazar, R. D. O'Neill, M. Martin, R. Roche and J. L. Gonzalez-Mora, Sens. Actuators, B, 2011, 152, 137–143 CrossRef CAS.
- Y. Tan, W. Deng, C. Chen, Q. Xie, L. Lei, Y. Li, Z. Fang, M. Ma, J. Chen and S. Yao, Biosens. Bioelectron., 2010, 25, 2644–2650 CrossRef CAS PubMed.
- G. S. Wilson and R. Gifford, Biosens. Bioelectron., 2005, 20, 2388–2403 CrossRef CAS PubMed.
- K. Balasubramanian, Biosens. Bioelectronic., 2010, 26, 1195–1204 CrossRef CAS PubMed.
- C. P. Andrieux and J. M. Savéant, Chem. Rev., 1990, 90, 723–738 CrossRef CAS.
- D. Chen, L. H. Tang and J. H. Li, Chem. Soc. Rev., 2010, 39, 3157–3180 RSC.
- D. Li and R. B. Kaner, Science., 2008, 320, 1170–1171 CrossRef CAS PubMed.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS PubMed.
- X. Zhao, P. Zhang, Y. Chen, Z. Su and G. Wei, Nanoscale, 2015, 7, 5080–5093 RSC.
- J. Ding, S. Zhu, T. Zhu, W. Sun, Q. Li, G. Wei and Z. Su, RSC Adv., 2015, 5, 22935–22942 RSC.
- J. Ding, W. Sun, G. Wei and Z. Su, RSC Adv., 2015, 5, 35338–35345 RSC.
- P. Zhang, Y. Huang, X. Lu, S. Zhang, J. Li, G. Wei and Z. Su, Langmuir, 2014, 30, 8980–8989 CrossRef CAS PubMed.
- P. Zhang, X. Zhang, S. Zhang, X. Lu, Q. Li, Z. Su and G. Wei, J. Mater. Chem. B, 2013, 1, 6525–6531 RSC.
- M. Pumera, A. Ambrosi, A. Bonanni, E. L. K. Chng and H. L. Poh, TrAC, Trends Anal. Chem., 2010, 29, 954–965 CrossRef CAS.
- Z. Qingbin, B. Zhang, X. Lin, X. Shen, N. Yousefi, Z. D. Huang, Z. Li and J. K. Kim, J. Mater. Chem., 2012, 22, 25072–25082 RSC.
- X. Li, G. Zhang, X. Bai, X. Sun, X. Wang, E. Wang and H. Dai, Nat. Nanotechnol., 2008, 3, 538–542 CrossRef CAS PubMed.
- D. S. Su and R. Schlögl, ChemSusChem, 2010, 3, 136–168 CrossRef CAS PubMed.
- H. C. Schniepp, J. L. Li, M. J. McAllister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud'homme, R. Car, D. A. Saville and I. A. Aksay, J. Phys. Chem. B, 2006, 110, 8535–8539 CrossRef CAS PubMed.
- H. Yin, Q. Zhang, Y. Zhou, Q. Ma, T. Liu, L. Zhu and S. Ai, Electrochim. Acta, 2011, 56, 2748–2753 CrossRef CAS.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771–778 CrossRef CAS.
- Y. Y. Chen, H. Y. Lai, S. H. Lin, C. W. Cho, W. H. Chao, C. H. Liao, S. Tsang, Y. F. Cheng and S. Y. Ling, J. Neurosci. Methods, 2009, 182, 6–16 CrossRef PubMed.
- L. Chen, Y. Tang, K. Wang, C. Liu and S. Luo, Electrochem. Commun., 2011, 13, 133–137 CrossRef CAS.
- R. P. Frankenthal and D. J. Siconolfi, J. Electrochem. Soc., 1982, 129, 1192–1194 CrossRef CAS.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 075414 CrossRef.
- N. Jia, Z. Wang, G. Yang, H. Shen and L. Z. Zhu, Electrochem. Commun., 2007, 9, 233–238 CrossRef CAS.
- F. Xu, M. Deng, G. Li, S. Chen and L. Wang, Electrochim. Acta, 2013, 88, 59–65 CrossRef CAS.
- M. Liu, R. Liu and W. Chen, Biosens. Bioelectron., 2013, 45, 206–212 CrossRef CAS PubMed.
- S. Palanisamy, S. M. Chen and R. Sarawathi, Sens. Actuators, B, 2012, 166–167, 372–377 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |