Evaluation of some quinoline-based hydrazone derivatives as insecticidal agents†
Xiang Yua,
Gang Fengb,
Jiulin Huanga and
Hui Xu*a
aResearch Institute of Pesticidal Design & Synthesis, College of Sciences, Northwest A&F University, Yangling 712100, Shaanxi Province, People′s Republic of China. E-mail: orgxuhui@nwsuaf.edu.cn; Fax: +86-29-87091952; Tel: +86-29-87091952
bEnvironment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Science, Haikou 571101, Hainan Province, People′s Republic of China
First published on 14th March 2016
Abstract
In continuation of our program aimed at the discovery and development of efficient insecticidal agents, a series of quinoline-based hydrazone derivatives were synthesized and evaluated as insecticidal agents against three-day-old larvae of Spodoptera litura (Noctuidae: Lepidoptera), a polyphagous insect pest of many important crops, in vivo at 1 mg mL−1. In particular, compounds 3c, 3e, 4g, 4h, and 6f showed potent insecticidal activity with 7 day mortality rates greater than 93%, and were comparable to that of the positive control toosendanin.
Introduction
Spodoptera litura (Noctuidae: Lepidoptera) is a polyphagous insect pest of many important crops such as cotton, vegetables, soybean, cauliflower, castor and others.1 Outbreaks of S. litura are frequently responsible for severe yield losses in many economically important crops, and recently various pesticides have been widely used to control S. litura. However, the extensive application of those agrochemicals over the years has led to the development of insecticide resistance in insect pest populations.2–6 Therefore, research and development of new pesticides to efficiently control S. litura is highly desirable.The quinoline skeleton (I, Fig. 1) occurs in various biologically active natural products and synthetic compounds, which exhibit a variety of interesting activities such as antiplasmodial activity,7 antimicrobial activity,8 anticancer activity,9 antiplasmodial activity,10 antimalarial activity,11 and antioxidant activity.12 Recently, we found that introduction of hydrazone fragments into podophyllotoxin, N-arylsulfonyl-3-acylindole or fraxinellone could result in more potent compounds as insecticidal agents, i.e., podophyllotoxin-based hydrazones (II, Fig. 1),13 N-arylsulfonyl-3-acylindole arylcarbonyl hydrazones (III, Fig. 1),14 and fraxinellone-based hydrazones (IV and V, Fig. 1),15 respectively. Encouraged by the above results, and in continuation of our program aimed at the discovery and development of novel pesticidal agents, here we prepared a series of quinoline-based hydrazone derivatives (VI, Fig. 1) by introduction of hydrazone moiety (Part A) into the quinoline skeleton (Part B). Their insecticidal activity was tested against the three-day-old larvae of S. litura in vivo.
Materials and methods
General procedure for synthesis of compounds 2a and 2b
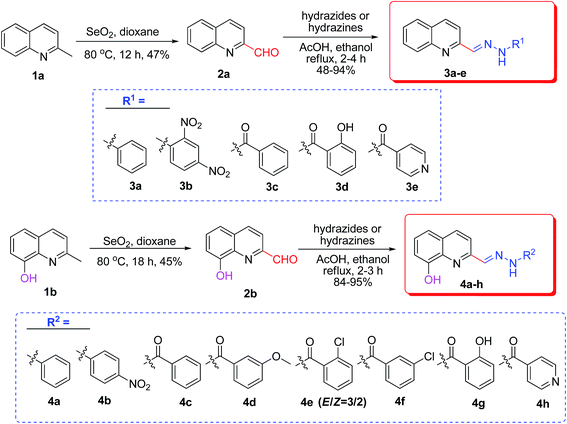
2-Methylquinoline (1a) or 8-hydroxyquinaldine (1b, 1 mmol) reacted with SeO2 (1.1 mmol) in dioxane (10 mL) at 80 °C. After 12 or 18 h, the mixture was cooled to room temperature. The precipitate was filtered and washed with CH2Cl2 (20 mL). The combined organic phases were concentrated and purified by preparative thin-layer chromatography (PTLC) to give compounds 2a [mp 68–70 °C (lit. 68 °C)16] or 2b [mp 94–96 °C (lit. 95–96 °C)17].Synthesis of compound 2c
To a mixture of 1b (1 mmol) and K2CO3 (2 mmol) in DMF (20 mL) at room temperature, CH3I (2 mmol) was added. Then the mixture was stirred at room temperature for 15 h. After the rest of CH3I in the mixture was removed by evaporation, the mixture was diluted by water (15 mL), and extracted with EtOAc (3 × 30 mL). The combined organic layer was dried over anhydrous Na2SO4, concentrated and purified by silica gel column chromatography (200–300 mesh) to afford compound 1c. Subsequently, the product 2c [mp 100–102 °C (lit. 103 °C)]18 was prepared from 1c by the same method as described above.Synthesis of compound 2d
To a solution of 1b (1 mmol) in CH2Cl2 (10 mL) at 0 °C, benzenesulfonyl chloride (1.2 mmol) and triethylamine (1.5 mmol) were added in sequence. After addition, the above mixture was stirred at room temperature for 30 h. The solvent was removed under reduced pressure to give the residue, which was purified by PTLC to give compound 1d. Subsequently, the product 2d was prepared from 1d by the same method as described above.Data for 2d
Yield = 85%, white solid, mp 152–154 °C; 1H NMR (400 MHz, DMSO-d6) δ: 9.80 (s, 1H), 8.29 (d, J = 8.4 Hz, 1H), 7.94–7.97 (m, 3H), 7.85 (dd, J = 8.0, 1.2 Hz, 1H), 7.80 (dd, J = 7.6, 1.6 Hz, 1H), 7.71 (t, J = 8.0 Hz, 1H), 7.59 (d, J = 7.6 Hz, 1H), 7.46 (t, J = 8.0 Hz, 2H).General procedure for synthesis of compounds 3a–e, 4a–h, 5a–e, and 6a–f
A mixture of 2a, 2b, 2c or 2d (1 mmol), hydrazides or hydrazines (1 mmol), and two drops of HOAc in EtOH (10 mL) was refluxed. When the reaction was complete checked by TLC after 2–4 h, the resulting reaction mixture was cooled to room temperature until no more precipitate was observed. The crude solid was then collected by filtration, and washed with cooled ethanol and petroleum ether in sequence to afford the target compounds 3a–e, 4a–h, 5a–e, or 6a–f.Data for 3a
CAS no. 7727-09-5. Yield = 91%, yellow solid, mp 202–204 °C (lit. 204 °C);19 IR cm−1 (KBr): 3445, 1598, 1563; 1H NMR (400 MHz, DMSO-d6) δ: 10.92 (s, 1H), 8.29 (d, J = 8.8 Hz, 1H), 8.12 (d, J = 8.8 Hz, 1H), 8.04 (s, 1H), 7.93–7.97 (m, 2H), 7.71–7.75 (m, 1H), 7.53–7.57 (m, 1H), 7.27–7.31 (m, 2H), 7.18–7.21 (m, 2H), 6.83 (t, J = 7.2 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 155.60, 147.98, 144.99, 137.27, 136.50, 130.21, 129.76, 128.90, 128.36, 127.31, 126.68, 120.32, 117.72, 112.99; HRMS m/z calcd for C16H14N3 ([M + H]+) 248.1182, found 248.1181.Data for 3b
CAS no. 14148-37-9. Yield = 84%, yellow solid, mp 246–248 °C (lit. 247–248 °C);20 IR cm−1 (KBr): 3446, 1616, 1583, 1336, 1321; 1H NMR (500 MHz, DMSO-d6) δ: 11.81 (s, 1H), 8.86 (s, 1H), 8.80 (s, 1H), 8.39–8.42 (m, 2H), 8.19–8.23 (m, 2H), 8.03 (d, J = 8.5 Hz, 1H), 7.98 (d, J = 8.0 Hz, 1H), 7.78–7.80 (m, 1H), 7.62–7.65 (m, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 153.82, 149.38, 148.03, 144.69, 138.59, 137.16, 131.14, 130.54, 130.10, 129.48, 128.44, 128.41, 127.89, 123.14, 118.32, 117.72; HRMS m/z calcd for C16H12O4N5 ([M + H]+) 338.0884, found 338.0881.Data for 3c
CAS no. 106869-10-7. Yield = 91%, yellow solid, mp 168–170 °C (lit. 187 °C);21 IR cm−1 (KBr): 3445, 1674, 1621, 1585; 1H NMR (400 MHz, DMSO-d6) δ: 8.66 (d, J = 8.8 Hz, 1H), 8.02–8.11 (m, 4H), 7.91–7.97 (m, 3H), 7.72–7.79 (m, 4H); 13C NMR (125 MHz, DMSO-d6) δ: 164.02, 154.31, 148.46, 147.87, 137.25, 133.69, 132.53, 130.58, 129.40, 129.08, 128.51, 128.41, 128.27, 127.82, 118.00; HRMS m/z calcd for C17H14ON3 ([M + H]+) 276.1131, found 276.1130.Data for 3d
CAS no. 894695-24-0. Yield = 95%, white solid, mp 265–266 °C (lit. not report);22 IR cm−1 (KBr): 3450, 3243, 1657, 1607, 1545, 1150; 1H NMR (400 MHz, DMSO-d6) δ: 12.13 (s, 1H), 11.60 (s, 1H), 8.62 (s, 1H), 8.44 (d, J = 8.8 Hz, 1H), 8.13 (d, J = 8.4 Hz, 1H), 8.02–8.07 (m, 2H), 7.87–7.89 (m, 1H), 7.79 (t, J = 7.2 Hz, 1H), 7.64 (t, J = 7.6 Hz, 1H), 7.45 (t, J = 7.2 Hz, 1H), 6.97–7.02 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ: 165.40, 159.12, 154.14, 149.04, 147.88, 137.31, 134.39, 130.62, 129.42, 129.38, 128.53, 128.45, 127.91, 119.55, 118.06, 117.71, 117.04; HRMS m/z calcd for C17H14O2N3 ([M + H]+) 292.1081, found 292.1079.Data for 3e
CAS no. 92869-04-0. Yield = 94%, white solid, mp 194–196 °C (lit. 197 °C);22 IR cm−1 (KBr): 3428, 1662, 1598, 1558; 1H NMR (400 MHz, DMSO-d6) δ: 12.42 (s, 1H), 8.82–8.84 (m, 2H), 8.63 (s, 1H), 8.45 (d, J = 8.8 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.02–8.08 (m, 2H), 7.86–7.88 (m, 2H), 7.80–7.84 (m, 1H), 7.65–7.68 (m, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 162.52, 153.98, 150.92, 149.65, 147.86, 140.70, 137.38, 130.65, 129.46, 128.53, 128.50, 127.99, 122.10, 118.03; HRMS m/z calcd for C16H13ON4 ([M + H]+) 276.1032, found 276.1030.Data for 4a
Yield = 72%, yellow solid, mp 164–166 °C; IR cm−1 (KBr): 3361, 3287, 1598, 1566, 1250; 1H NMR (500 MHz, DMSO-d6) δ: 10.94 (s, 1H), 9.62 (s, 1H), 8.23 (d, J = 9.0 Hz, 1H), 8.12 (d, J = 8.5 Hz, 1H), 8.10 (s, 1H), 7.35–7.40 (m, 2H), 7.28 (t, J = 7.5 Hz, 2H), 7.20 (d, J = 7.5 Hz, 2H), 7.08 (d, J = 6.5 Hz, 1H), 6.83 (t, J = 7.5 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 153.59, 144.91, 138.40, 137.25, 136.45, 129.76, 128.47, 127.57, 120.26, 118.27, 117.97, 112.96, 112.92, 112.15; HRMS m/z calcd for C16H14ON3 ([M + H]+) 264.1131, found 264.1129.Data for 4b
Yield = 72%, yellow solid, mp 280–282 °C; IR cm−1 (KBr): 3399, 3266, 1609, 1576, 1316, 1248; 1H NMR (400 MHz, DMSO-d6) δ: 11.71 (s, 1H), 9.75 (s, 1H), 8.30 (d, J = 8.4 Hz, 1H), 8.25 (s, 1H), 8.16–8.21 (m, 3H), 7.38–7.46 (m, 2H), 7.30 (d, J = 8.8 Hz, 2H), 7.11 (dd, J = 7.2, 1.6 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 153.32, 152.12, 150.19, 141.96, 139.42, 138.21, 136.41, 128.54, 127.94, 126.21, 117.90, 117.77, 112.09; HRMS m/z calcd for C16H13O3N4 ([M + H]+) 309.0982, found 309.0981.Data for 4c
CAS no. 439294-17-4. Yield = 82%, pale yellow solid, mp 222–224 °C (lit. 221 °C);23 IR cm−1 (KBr): 3359, 3318, 1684, 1602, 1546, 1266; 1H NMR (500 MHz, DMSO-d6) δ: 12.27 (s, 1H), 9.88 (s, 1H), 8.68 (s, 1H), 8.36 (d, J = 8.5 Hz, 1H), 8.12 (d, J = 8.5 Hz, 1H), 7.97 (d, J = 7.0 Hz, 2H), 7.63 (t, J = 7.0 Hz, 1H), 7.56–7.59 (m, 2H), 7.42–7.49 (m, 2H), 7.15 (d, J = 7.5 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 164.05, 153.91, 152.23, 148.37, 138.60, 137.09, 133.72, 132.50, 129.32, 129.05, 128.80, 128.29, 118.31, 118.18, 112.66; HRMS m/z calcd for C17H14O2N3 ([M + H]+) 292.1081, found 292.1079.Data for 4d
Yield = 51%, white solid, mp 166–168 °C; IR cm−1 (KBr): 3408, 3183, 2834, 1649, 1585, 1557, 1373, 1128; 1H NMR (400 MHz, DMSO-d6) δ: 12.21 (s, 1H), 9.85 (s, 1H), 8.67 (s, 1H), 8.35 (d, J = 8.8 Hz, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.41–7.55 (m, 5H), 7.20 (d, J = 8.0 Hz, 1H), 7.14 (d, J = 6.8 Hz, 1H), 3.86 (s, 3H) 13C NMR (125 MHz, DMSO-d6) δ: 163.70, 159.76, 153.90, 153.73, 152.23, 148.50, 138.55, 137.11, 135.07, 130.27, 129.32, 128.81, 120.46, 118.37, 118.18, 113.62, 112.55; 55.89; HRMS m/z calcd for C18H16O3N3 ([M + H]+) 322.1186, found 322.1185.Data for 4e (E/Z = 3/2)
Yield = 74%, pale yellow solid, mp 192–194 °C; IR cm−1 (KBr): 3427, 3173, 1666, 1594, 1566, 1373, 1129; 1H NMR (400 MHz, CDCl3) δ: 12.43 (s, 0.2H), 12.32 (s, 0.3H), 9.90 (s, 0.3H), 9.87 (s, 0.2H), 8.49 (s, 0.6H), 8.36 (d, J = 8.8 Hz, 0.6H), 8.30 (s, 0.4H), 8.18 (d, J = 8.4 Hz, 0.4H), 8.10 (d, J = 8.8 Hz, 0.6H), 7.31–7.67 (m, 6.4H), 7.10–7.15 (m, 1H); HRMS m/z calcd for C17H13O2N3Cl ([M + H]+) 326.0691, found 326.0686.Data for 4f
Yield = 91%, white solid, mp 168–170 °C; IR cm−1 (KBr): 3428, 3277, 1665, 1651, 1553, 1379, 1087; 1H NMR (400 MHz, DMSO-d6) δ: 12.32 (s, 1H), 9.86 (s, 1H), 8.65 (s, 1H), 8.36 (d, J = 8.8 Hz, 1H), 8.11 (d, J = 8.8 Hz, 1H), 8.01 (s, 1H), 7.92 (d, J = 7.2 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.59 (t, J = 8.0 Hz, 1H), 7.42–7.50 (m, 2H), 7.14 (d, J = 7.2 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 162.55, 153.77, 152.09, 148.92, 138.55, 137.18, 135.64, 133.85, 132.35, 131.10, 129.38, 128.90, 128.00, 127.16, 118.36, 118.23, 112.64; HRMS m/z calcd for C17H13O2N3Cl ([M + H]+) 326.0691, found 326.0689.Data for 4g
CAS no. 1184997-85-0. Yield = 73%, yellow solid, mp 246–248 °C (lit. 245–247 °C);23 IR cm−1 (KBr): 3465, 3252, 1644, 1608, 1532, 1224; 1H NMR (400 MHz, DMSO-d6) δ: 12.18 (s, 1H), 11.64 (s, 1H), 9.87 (s, 1H), 8.65 (s, 1H), 8.36 (d, J = 8.4 Hz, 1H), 8.12 (d, J = 8.8 Hz, 1H), 7.89 (d, J = 7.2 Hz, 1H), 7.42–7.50 (m, 3H), 7.15 (d, J = 7.2 Hz, 1H), 6.98–7.03 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ: 165.49, 159.18, 153.95, 152.02, 148.98, 138.64, 137.16, 134.37, 129.38, 129.35, 128.92, 119.52, 118.33, 118.22, 117.72, 117.04, 112.68; HRMS m/z calcd for C17H14O3N3 ([M + H]+) 308.1030, found 308.1024.Data for 4h
CAS no. 298218-46-9. Yield = 71%, yellow solid, mp 160–162 °C (lit. 162–164 °C);23 IR cm−1 (KBr): 3446, 1679, 1616, 1548, 1281; 1H NMR (400 MHz, DMSO-d6) δ: 12.46 (s, 1H), 9.90 (s, 1H), 8.83 (d, J = 7.0 Hz, 2H), 8.67 (s, 1H), 8.37 (d, J = 8.8 Hz, 1H), 8.12 (d, J = 8.8 Hz, 1H), 7.87 (d, J = 6.8 Hz, 2H), 7.42–7.51 (m, 2H), 7.15 (d, J = 7.2 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 162.60, 153.42, 151.04, 150.69, 140.53, 139.71, 138.74, 137.40, 129.64, 129.10, 123.86, 122.31, 118.53, 113.45; HRMS m/z calcd for C16H13O2N4 ([M + H]+) 293.1033, found 293.1031.Data for 5a
Yield = 74%, yellow solid, mp 108–110 °C; IR cm−1 (KBr): 3445, 2916, 1615, 1553, 1384, 1121; 1H NMR (500 MHz, DMSO-d6) δ: 15.49 (s, 1H), 8.41 (d, J = 8.5 Hz, 1H), 7.69 (d, J = 8.5 Hz, 1H), 7.573 (t, J = 8.0 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.46 (s, 1H), 7.36 (t, J = 7.5 Hz, 2H), 7.31 (d, J = 8.0 Hz, 1H), 7.26 (d, J = 8.0 Hz, 2H), 6.92 (t, J = 7.5 Hz, 1H), 4.15 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ: 154.34, 152.20, 144.74, 137.33, 137.25, 130.07, 128.39, 128.01, 127.58, 122.44, 121.30, 119.58, 113.26, 109.53, 56.68; HRMS m/z calcd for C17H16ON3 ([M + H]+) 278.1288, found 278.1286.Data for 5b
Yield = 72%, white solid, mp 206–208 °C; IR cm−1 (KBr): 3449, 2834, 1660, 1604, 1558, 1377, 1105; 1H NMR (400 MHz, DMSO-d6) δ: 12.18 (s, 1H), 8.62 (s, 1H), 8.37 (d, J = 8.8 Hz, 1H), 8.13 (d, J = 8.4 Hz, 1H), 7.94 (d, J = 7.6 Hz, 2H), 7.54–7.64 (m, 5H), 7.23 (d, J = 7.2 Hz, 1H), 4.00 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ: 164.09, 155.65, 152.83, 148.58, 139.63, 137.03, 133.82, 132.49, 129.46, 129.10, 128.30, 128.26, 119.90, 118.35, 109.54, 56.18; HRMS m/z calcd for C18H16O2N3 ([M + H]+) 306.1237, found 306.1235.Data for 5c (E/Z = 3/2)
Yield = 81%, white solid, mp 220–221 °C; IR cm−1 (KBr): 3449, 2838, 1694, 1591, 1552, 1378, 1105; 1H NMR (400 MHz, CDCl3) δ: 12.38 (s, 0.3H), 12.28 (s, 0.45H), 18.43 (s, 0.6H), 8.38 (d, J = 8.8 Hz, 0.6H), 8.25 (s, 0.4H), 8.19 (d, J = 8.8 Hz, 0.4H), 8.12 (d, J = 8.8 Hz, 0.6H), 7.40–7.67 (m, 6.4H), 7.20–7.25 (m, 1H), 3.99 (s, 1.8H), 3.97 (s, 1.2H); HRMS m/z calcd for C18H15O2N3Cl ([M + H]+) 340.0847, found 340.0844.Data for 5d
Yield = 58%, white solid, mp 176–178 °C; IR cm−1 (KBr): 3446, 2836, 1666, 1614, 1562, 1378, 1106; 1H NMR (400 MHz, DMSO-d6) δ: 12.25 (s, 1H), 8.60 (s, 1H), 8.37 (d, J = 8.8 Hz, 1H), 8.13 (d, J = 8.4 Hz, 1H), 7.99 (s, 1H), 7.90 (d, J = 7.6 Hz, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.55–7.64 (m, 3H), 7.23 (dd, J = 7.2, 1.2 Hz, 1H), 4.00 (s, 3H); 162.66, 155.69, 152.68, 149.18, 139.67, 137.07, 135.83, 133.88, 132.31, 131.13, 129.52, 128.37, 127.95, 127.10, 119.92, 118.37, 109.62, 56.23; HRMS m/z calcd for C18H15O2N3Cl ([M + H]+) 340.0847, found 340.0845.Data for 5e (E/Z = 2/1)
Yield = 93%, yellow solid, mp 232–234 °C; IR cm−1 (KBr): 3446, 2830, 1647, 1618, 1589, 1384, 1137; 1H NMR (400 MHz, CDCl3) δ: 15.63 (s, 0.6H), 12.07 (s, 0.3H), 11.78 (s, 0.6H), 11.55 (s, 0.3H), 8.60 (d, J = 8.4 Hz, 1H), 8.37 (d, J = 8.8 Hz, 0.3H), 7.85–8.14 (m, 2.6H), 7.44–7.69 (m, 3H), 7.35 (d, J = 7.6 Hz, 0.7H), 7.23 (d, J = 7.6 Hz, 0.34H), 6.97–7.12 (m, 2H), 4.00 (s, 1H), 3.94 (s, 2H); HRMS m/z calcd for C18H16O3N3 ([M + H]+) 322.1186, found 322.1185.Data for 6a
Yield = 65%, yellow solid, mp 141–142 °C; IR cm−1 (KBr): 3447, 1598, 1568, 1244, 1184; 1H NMR (400 MHz, DMSO-d6) δ: 10.97 (s, 1H), 8.27 (d, J = 8.8 Hz, 1H), 8.07 (d, J = 8.8 Hz, 1H), 7.90–7.94 (m, 3H), 7.77 (s, 1H), 7.71–7.50 (m, 1H), 7.53–7.61 (m, 4H), 7.28–7.32 (m, 2H), 7.19 (d, J = 7.6 Hz, 2H), 6.85 (t, J = 7.6 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ: 155.86, 144.79, 144.64, 140.84, 136.74, 136.40, 135.70, 135.12, 129.78, 129.68, 129.09, 128.91, 127.89, 126.22, 123.57, 120.58, 118.48, 113.12; HRMS m/z calcd for C22H18O3N3S ([M + H]+) 404.1063, found 404.1062.Data for 6b
Yield = 72%, yellow solid, mp 260–262 °C; IR cm−1 (KBr): 3445, 1616, 1581, 1312, 1271, 1161; 1H NMR (500 MHz, DMSO-d6) δ: 12.03 (s, 1H), 9.08 (d, J = 2.0 Hz, 1H), 8.83 (s, 1H), 8.59–8.62 (m, 2H), 8.38–8.40 (m, 2H), 8.183–8.16 (m, 3H), 7.83–7.88 (m, 3H), 7.75–7.79 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ: 154.06, 148.72, 145.12, 144.59, 140.93, 138.79, 137.18, 135.82, 134.98, 131.34, 130.09, 129.74, 129.69, 128.89, 127.85, 127.53, 123.49, 123.12, 119.05, 117.78; HRMS m/z calcd for C22H16O7N5S ([M + H]+) 494.0765, found 494.0762.Data for 6c
Yield = 67%, white solid, mp 200–201 °C; IR cm−1 (KBr): 3447, 1657, 1593, 1572, 1171; 1H NMR (400 MHz, DMSO-d6) δ: 12.22 (s, 1H), 8.43 (d, J = 9.2 Hz, 2H), 8.07 (d, J = 8.8 Hz, 1H), 7.97–8.00 (m, 3H), 7.89 (d, J = 7.6 Hz, 2H), 7.70 (t, J = 7.6 Hz, 1H), 7.57–7.61 (m, 7H); 13C NMR (125 MHz, DMSO-d6) δ: 163.94, 154.50, 147.95, 144.88, 140.77, 137.31, 135.41, 135.23, 133.56, 132.65, 129.80, 129.72, 129.12, 128.91, 128.32, 128.08, 127.54, 123.80, 118.70; HRMS m/z calcd for C23H18O4N3S ([M + H]+) 432.1013, found 432.1004.Data for 6d (E/Z = 3/2)
Yield = 74%, white solid, mp 199–200 °C; IR cm−1 (KBr): 3449, 1664, 1590, 1554, 1189; 1H NMR (400 MHz, DMSO-d6) δ: 12.42 (s, 0.4H), 12.32 (s, 0.6H), 8.44 (d, J = 8.8 Hz, 0.6H), 8.26 (d, J = 8.8 Hz, 0.4H), 8.17 (s, 0.6H), 8.06 (d, J = 8.8 Hz, 0.6H), 8.00–8.02 (m, 0.6H), 7.97 (s, 0.4H), 7.87–7.89 (m, 2.4H), 7.47–7.73 (m, 9H), 7.32 (d, J = 8.4 Hz, 0.4H); HRMS m/z calcd for C23H17O4N3ClS ([M + H]+) 466.0623, found 466.0620.Data for 6e
Yield = 78%, white solid, mp 206–208 °C; IR cm−1 (KBr): 3445, 3277, 1640, 1609, 1533, 1228, 1160; 1H NMR (400 MHz, DMSO-d6) δ: 8.44 (d, J = 8.4 Hz, 1H), 8.37 (s, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.99 (dd, J = 7.2, 1.6 Hz, 1H), 7.90–7.92 (m, 3H), 7.70 (t, J = 7.6 Hz, 1H), 7.64–7.67 (m, 2H), 7.56–7.60 (m, 2H), 7.47 (t, J = 7.6 Hz, 1H), 7.00–7.05 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ: 165.29, 158.96, 154.31, 148.39, 144.92, 140.74, 137.37, 135.44, 135.20, 134.43, 129.79, 129.74, 129.53, 128.96, 128.10, 127.63, 123.89, 119.63, 118.75, 117.71, 117.20; HRMS m/z calcd for C23H18O5N3S ([M + H]+) 448.0962, found 448.0963.Data for 6f
Yield = 76%, white solid, mp 230–232 °C; IR cm−1 (KBr): 3444, 3340, 1650, 1609, 1548, 1275, 1179; 1H NMR (400 MHz, DMSO-d6) δ: 8.41 (d, J = 8.8 Hz, 2H), 8.05 (d, J = 6.0 Hz, 1H), 7.97 (dd, J = 7.6, 2.0 Hz, 1H), 7.87–7.91 (m, 4H), 7.70 (t, J = 7.6 Hz, 1H), 7.57–7.67 (m, 4H), 6.92 (d, J = 8.4 Hz, 2H); 13C NMR (125 MHz, DMSO-d6) δ: 163.56, 161.52, 154.70, 144.86, 140.77, 137.21, 135.41, 135.21, 130.56, 130.50, 129.79, 129.66, 128.90, 128.06, 127.41, 123.93, 123.74, 118.66, 115.59; HRMS m/z calcd for C23H18O5N3S ([M + H]+) 448.0962, found 448.0961.Biological assay
The insecticidal activity of compounds 3a–e, 4a–h, 5a–e and 6a–f against the three-day-old larvae of Spodoptera litura was assessed by leaf-dipping method.14 Toosendanin, isolated from Melia azedarach, was used as a positive control. For each compound, 30 three-day-old larvae (10 larvae per group) were used. Acetone solutions of all the above tested compounds, and toosendanin were prepared at the concentration of 1 mg mL−1. Small circular leaf discs were cut from fresh castor leaves using a cork borer (1.0 cm in diameter) and dipped into the corresponding solution for 5 s, then taken out and dried in a room. Leaf discs treated with acetone alone were used as a blank control group. Several treated leaf discs were kept in each dish (9.0 cm in diameter), where every 10 larvae were raised. If the treated leaves were consumed, additional treated leaves were added to the dish. After 48 h, compound-soaked leaves were removed, and the larvae were fed with untreated fresh wheat leaves thereafter. The experiment was carried out at 28 ± 2 °C and on 16 h/8 h (light/dark) photoperiod. The insecticidal activities of the tested compounds against the three-day-old larvae of S. litura after 2, 3 and 7 days were calculated by the following formula:| Corrected mortality rate (%) = (T − C) × 100/(100% − C) |
Results and discussion
Synthesis
As shown in Scheme 1, first, oxidation of 2-methylquinoline (1a) or 8-hydroxyquinaldine (1b) with SeO2 gave 2-formylquinoline (2a)16 and 2-formyl-8-hydroxyquinoline (2b),17 respectively. Then compounds 2a and 2b reacted with hydrazines or hydrazides to afford quinoline-based hydrazones 3a–e and 4a–h. Another quinoline-based hydrazones 5a–e and 6a–f were prepared as follows (Scheme 2): compound 1b firstly reacted with methyl iodide or benzenesulfonyl chloride to give 2-methyl-8-methoxyquinoline (1c) and 2-methyl-8-benzenesulfonyloxyquinoline (1d), respectively. Subsequently, compounds 1c and 1d were oxidized by SeO2 to produce 2-formyl-8-methoxyquinoline (2c)18 and 2-formyl-8-benzenesulfonyloxyquinoline (2d), respectively. Finally, compounds 2c and 2d reacted with hydrazines or hydrazides to afford 5a–e and 6a–f.Insecticidal activity
The insecticidal activity of compounds 3a–e, 4a–h, 5a–e and 6a–f against the three-day-old larvae of S. litura was evaluated by leaf-dipping method at a concentration of 1 mg mL−1. Toosendanin, a commercial botanical insecticide isolated from Melia azedarach, was used as the positive control at 1 mg mL−1. Leaves treated with acetone alone were used as a blank control group. As shown in Table 1, compounds 3c, 3e, 4g, 4h, and 6f showed potent insecticidal activity with 7 day mortality rates greater than 93%, and were comparable to that of toosendanin. Additionally, their structure–activity relationships were also observed. Among compounds 3a–e, 4a–h and 5a–e, the hydrazine derivatives were generally less active compared with the corresponding hydrazide ones (e.g., 3a vs. 3c; 4a vs. 4c; 5a vs. 5c). To derivatives 3a–e, compounds 3c (containing benzoylhydrazone moiety) and 3e (containing pyrid-4-ylcarbonylhydrazone moiety) exhibited potent insecticidal activity with 7 day mortality rates of 96.6%. Whereas introduction of a hydroxyl group on the phenyl ring of 3c led to less active compound 3d (7 day mortality rate: 69%). To compounds 4a–h, introduction of 2-hydroxybenzoylhydrazone or pyrid-4-ylcarbonylhydrazone moiety into 8-hydroxyquinoline afforded potent derivatives 4g and 4h with 7 day mortality rates of 96.6%, and 100%, respectively. Obviously, once pyrid-4-ylcarbonylhydrazone moiety was introduced into quinoline or 8-hydroxyquinoline, the corresponding compounds 3e and 4h all showed the potent insecticidal activity. However, all 8-methoxyquinoline hydrazones (5a–e) displayed less potent insecticidal activity. It demonstrated that to obtain the promising quinoline-based hydrazone derivatives, the hydroxyl group of 8-hydroxyquinoline should not be methylated. Among compounds 6a–f, 4-hydroxybenzoylhydrazone derivative 6f exhibited the most potent insecticidal activity with the 7 day mortality rate of 93.1%; whereas the 7 day mortality rates of 6c–e were 55.2%, 72.4%, and 82.8%, respectively. It suggested, for 8-benzenesulfonyloxyquinoline-based benzoylhydrazone derivatives, introduction of a hydroxyl group on the phenyl ring of 6c was necessary for obtaining potent compounds (e.g., 6c vs. 6e and 6f).| Compound | Corrected mortality rate (%) | ||
|---|---|---|---|
| 2 days | 3 days | 7 days | |
| 3a | 33.3 ± 5.8 | 43.3 ± 5.8 | 48.3 ± 10.0 |
| 3b | 6.7 ± 11.6 | 30.0 ± 17.3 | 51.7 ± 11.6 |
| 3c | 36.7 ± 5.8 | 50.0 ± 10.0 | 96.6 ± 5.8 |
| 3d | 6.7 ± 11.6 | 36.7 ± 11.6 | 69.0 ± 10.0 |
| 3e | 30.0 ± 10.0 | 83.3 ± 11.6 | 96.6 ± 5.8 |
| 4a | 0 ± 0 | 3.3 ± 5.8 | 20.7 ± 5.8 |
| 4b | 3.3 ± 5.8 | 6.7 ± 5.8 | 51.7 ± 11.6 |
| 4c | 0 ± 0 | 6.7 ± 11.6 | 58.6 ± 10.0 |
| 4d | 3.3 ± 5.8 | 6.7 ± 5.8 | 69.0 ± 10.0 |
| 4e | 20.0 ± 10.0 | 26.7 ± 5.8 | 65.5 ± 5.8 |
| 4f | 20.0 ± 10.0 | 33.3 ± 5.8 | 55.2 ± 11.6 |
| 4g | 16.7 ± 5.8 | 73.3 ± 11.6 | 96.6 ± 5.8 |
| 4h | 40.0 ± 20.0 | 76.7 ± 5.8 | 100.0 ± 0.0 |
| 5a | 3.3 ± 5.8 | 10.0 ± 17.3 | 38.0 ± 10.0 |
| 5b | 0 ± 0 | 0 ± 0 | 48.3 ± 0.0 |
| 5c | 3.3 ± 5.8 | 10.0 ± 10.0 | 65.5 ± 5.8 |
| 5d | 3.3 ± 5.8 | 16.7 ± 5.8 | 48.3 ± 10.0 |
| 5e | 0 ± 0 | 10.0 ± 10.0 | 62.1 ± 5.8 |
| 6a | 26.7 ± 11.6 | 43.3 ± 15.3 | 69.0 ± 10.0 |
| 6b | 26.7 ± 15.3 | 63.3 ± 5.8 | 82.8 ± 5.8 |
| 6c | 26.7 ± 11.6 | 36.7 ± 11.6 | 55.2 ± 5.8 |
| 6d | 43.3 ± 5.8 | 56.7 ± 5.8 | 72.4 ± 15.3 |
| 6e | 36.7 ± 5.8 | 53.3 ± 5.8 | 82.8 ± 5.8 |
| 6f | 23.3 ± 5.8 | 56.7 ± 5.8 | 93.1 ± 11.6 |
| Toosendanin | 90.0 ± 10.0 | 96.7 ± 5.8 | 100.0 ± 0 |
| Blank control | 0 ± 0 | 0 ± 0 | 3.3 ± 5.8 |
Conclusions
In summary, a series of quinoline-based hydrazones were prepared, and evaluated for their insecticidal activity against three-day-old larvae of S. litura. Especially compounds 3c, 3e, 4g, 4h, and 6f showed potent insecticidal activity with 7 day mortality rates greater than 93% at 1 mg mL−1. It suggested that the pyrid-4-ylcarbonylhydrazine derivatives could be hybridized with quinolines at the C-2 position for preparation of promising insecticidal candidates in the future.Acknowledgements
The present research was supported by National Natural Science Foundation of China (No. 31171896), and the Special Funds of Central Colleges Basic Scientific Research Operating Expenses (YQ2013008).Notes and references
- N. Abbas, S. S. A. Shad, M. Razaq, A. Waheed and M. Aslam, Crop Prot., 2014, 58, 49–54 CrossRef CAS
.
- R. L. Wang, J. Li, C. Staehelin, X. W. Xin, Y. J. Su and R. S. Zeng, J. Chem. Ecol., 2015, 41, 111–119 CrossRef CAS PubMed
.
- J. Y. Su, T. Lai and J. Li, Crop Prot., 2012, 42, 217–222 CrossRef CAS
.
- W. Q. Chen, Z. J. Song and H. H. Xu, Bioorg. Med. Chem. Lett., 2012, 22, 5819–5822 CrossRef CAS PubMed
.
- X. Yu, D. Shi, X. Zhi, Q. Li, X. Yao and H. Xu, RSC Adv., 2015, 5, 31700–31707 RSC
.
- X. H. Lv, J. J. Xiao, Z. L. Ren, M. J. Chu, P. Wang, X. F. Meng, D. D. Li and H. Q. Cao, RSC Adv., 2015, 5, 55179–55185 RSC
.
- A. Barteselli, S. Parapini, N. Basilico, D. Mommo and A. Sparatore, Bioorg. Med. Chem., 2014, 22, 5757–5765 CrossRef CAS PubMed
.
- N. C. Desai, G. M. Kotadiya and A. R. Trivedi, Bioorg. Med. Chem. Lett., 2014, 24, 3126–3130 CrossRef CAS PubMed
.
- M. Chen, H. Chen, J. W. Ma, X. Y. Liu and S. Y. Zhang, Bioorg. Med. Chem. Lett., 2014, 24, 2867–2870 CrossRef CAS PubMed
.
- K. Singh, H. Kaur, P. Smith, C. de Kock, K. Chibale and J. Balzarini, J. Med. Chem., 2014, 57, 435–448 CrossRef CAS PubMed
.
- M. C. Joshi, K. J. Wicht, D. Taylor, R. Hunter, P. J. Smith and T. J. Egan, Eur. J. Med. Chem., 2013, 69, 338–347 CrossRef CAS PubMed
.
- Y. Zhang, Y. L. Fang, H. Liang, H. S. Wang, K. Hu, X. X. Liu, X. H. Yi and Y. Peng, Bioorg. Med. Chem. Lett., 2013, 23, 107–111 CrossRef CAS PubMed
.
- Y. Wang, X. Yu, X. Y. Zhi, X. Xiao, C. Yang and H. Xu, Bioorg. Med. Chem. Lett., 2014, 24, 2621–2624 CrossRef CAS PubMed
.
- Z. P. Che, S. Zhang, Y. Shao, L. L. Fan, H. Xu, X. Yu, X. Y. Zhi, X. Yao and R. Zhang, J. Agric. Food Chem., 2013, 61, 5696–5705 CrossRef CAS PubMed
.
- Y. Guo, Y. Y. Yan, C. Yang, X. Yu, X. Y. Zhi and H. Xu, Bioorg. Med. Chem. Lett., 2012, 22, 5384–5387 CrossRef CAS PubMed
.
- T. M. R. Maria, M. E. S. Eusébio, J. A. Silva, A. J. F. N. Sobral, C. Cardoso, J. A. Paixao and M. R. Silva, J. Mol. Struct., 2012, 1030, 67–74 CrossRef CAS
.
- M. Hassai, W. Cai, D. C. Holley, J. P. Lineswala, B. R. Maharijan, G. R. Ebrahimian, H. Seradj, M. G. Stocksdale, F. Mohamadi, C. C. Marvin, J. M. Gerdes, H. D. Beall and M. Behforouz, J. Med. Chem., 2005, 48, 7733–7749 CrossRef PubMed
.
- M. Wilhelm and S. Walter, Chem. Ber., 1957, 90, 758–761 CrossRef
.
- L. Mester, J. Am. Chem. Soc., 1955, 77, 4301–4305 CrossRef CAS
.
- L. King and S. V. Abramo, J. Org. Chem., 1958, 23, 1609–1612 CrossRef CAS
.
- R. S. Sheeja, N. Mamgalam, M. R. Kurup, Y. Mary, K. Raju, H. T. Varghese and C. Panicker, J. Mol. Struct., 2010, 973, 36–46 CrossRef
.
- S. Adsule, V. Barve, D. Chen, F. Ahmed, D. Q. Ping, S. Padhye and F. Sarkar, J. Am. Chem. Soc., 2006, 49, 7242–7246 CAS
.
- L. Yong-chun and Y. Zheng-yin, Eur. J. Med. Chem., 2009, 44, 5080–5089 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR and 13C-NMR spectra for the some typical target compounds. See DOI: 10.1039/c6ra00993j |
| This journal is © The Royal Society of Chemistry 2016 |