Fluorescent genipin cross-linked REDV-conjugated polymeric microbubbles for human vascular endothelial cell (HVEC) targeting†
Abstract
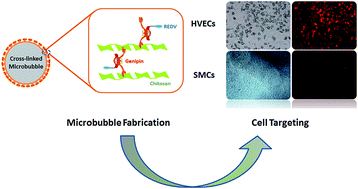
Polymeric microbubbles modified with chitosan and genipin were fabricated via emulsification, electrostatic interaction and cross-linking to afford intrinsic fluorescence. REDV peptides were conjugated to achieve HVEC active targeting. The degradation, cytotoxicity and targeting features made them potential candidates in early molecular diagnosis for cardiovascular diseases.

 Please wait while we load your content...
Please wait while we load your content...