DOI:
10.1039/C6RA00917D
(Paper)
RSC Adv., 2016,
6, 23537-23549
Morphology-dependent photoelectrochemical properties of multi-scale layered Bi(C2O4)OH†
Received
12th January 2016
, Accepted 24th February 2016
First published on 25th February 2016
Abstract
In this work, a facile hydrothermal process is employed to synthesize multi-scale layered Bi(C2O4)OH compounds (microrods, nanorods, nanowires and straw sheaves), which can be well controlled by simply tuning oxalate source, ethylene glycol/water (EG/W) volumetric ratio, H2C2O4/Bi(NO3)3 molar ratio, hydrothermal temperature and duration. Further, we have mainly investigated the transformation process from one-dimensional (1D) nanostructures to 3D straw sheaves of Bi(C2O4)OH. Moreover, it is found that the photoelectrochemical properties of Bi(C2O4)OH are greatly morphology-dependent: for the degradation of rhodamine B (RhB), on the one hand, the photocatalytic activity of nanorods is 2.45, 1.79 and 1.46 times higher than those of microrods, nanowires and straw sheaves, respectively; that is, the apparent reaction rate constants (k) are 0.0053, 0.01298, 0.00726, and 0.00888 min−1 for microrods, nanorods, nanowires and straw sheaves, respectively. On the other hand, the capacitance values of nanorods, microrods, nanowires and straw sheaves samples are 21.00, 19.00, 20.37 and 17.50 mF cm−2, respectively, which is in agreement with their photocatalytic activities.
1. Introduction
To date, various micro-/nanostructures have been applied in many fields, such as biological labeling and imaging, catalysis,1,2 drug delivery,3,4 sensing,5,6 surface-enhanced Raman scattering,7–10 and so on. The shape, size, and structure have great influences on the physicochemical properties of materials. Hence great attention has been paid to the controllable synthesis of new nanostructures. In particular, one dimensional (1D) to three-dimensional (3D) hierarchical architectures have attracted intensive attention.11–16 For example, many experimental observations and theoretical calculations have demonstrated that anisotropic metal nanostructures exhibit shape-dependent optical properties.17–19 Recently, our group have reported a series of new photocatalysts, including non-overlapped Ag3PO4 tetrapods,20 three-dimensional Ag3PO4 towers,21 CuO straw sheaves,22 six-branch- and snowflake-like BiPO4,23 etc. We have demonstrated that the photocatalytic activity closely interrelates with the size and shape of photocatalyst.19–23
Recently, bismuth containing compounds as a new type of photocatalytic materials have received considerable attention due to their incomparable oxidation ability. Bi2O2(OH)(NO3),24 Bi4B2O9,25 Bi2O2[BO2(OH)]25 and other newly found photocatalyst (Bi(C2O4)OH)26 all exhibit highly efficient photodegradation efficiency for removing contaminants in aqueous solution. Xiao et al.26 have reported that Bi(C2O4)OH, has an indirect band gap semiconductor (Eg = 3.73 eV), which could be a newly discovered, promising photocatalyst owing to its intrinsic physical and chemical properties, nontoxicity, low cost and high photocatalytic efficiency.26 To the best of our knowledge, however, only two work involves the synthesis of Bi(C2O4)OH, i.e. using precipitation route26 and sol–gel method.27 Nevertheless, the as-obtained Bi(C2O4)OH products are highly agglomerated, irregular particles.26,27 On one hand, the sol–gel method generally require rigorous operation conditions or intricate instruments, thus it is highly energy-consuming and not easy to scale up. On the other hand, it is difficult to finely control the particle morphology for the precipitation route. Thus, it is desirable to develop a simple, environmentally benign approach to synthesize Bi(C2O4)OH. To the best of our knowledge, little work has been done to obtain Bi(C2O4)OH with well-controlled new nanostructures.
In this work, new Bi(C2O4)OH multi-scale structures was, for the first time, prepared by a simple, mild hydrothermal method. We have investigated the influences of oxalate source, the volumetric ratio of EG to W, the molar ratio of H2C2O4/Bi(NO3)3, hydrothermal temperature and duration on the samples. Our studies show that new Bi(C2O4)OH nanostructures can be well controlled merely by tuning experimental parameters. A formation mechanism of Bi(C2O4)OH has also been proposed on base of the time-dependent results. Moreover, we have mainly investigated the morphology-dependent photoelectrochemistry properties of Bi(C2O4)OH.
2. Experimental
2.1 Sample preparation
All reagents were of analytical grade, purchased from Beijing Chemical Reagents Industrial Company of China, and were used without further purification.
In a typical experiment, Bi(NO3)3·5H2O and the measured amounts of oxalate source (H2C2O4·2H2O, (NH4)2C2O4·H2O, Na2C2O4) and 40 mL of distilled water were mixed together in a Teflon-lined stainless steel autoclave with a capacity of 50 mL. The autoclave was then kept at 120 °C for 12 h. When the reaction was completed, the autoclave was cooled to room temperature naturally. The white product was harvested by centrifugation, washed with ethanol and deionized water for several times, and dried overnight at 60 °C for 12 h.
In order to investigate the influences of preparation conditions on the samples, the hydrothermal temperature, duration, the volumetric ratio of ethylene glycol (EG) to water (W) and the molar ratio of H2C2O4/Bi(NO3)3, were varied.
2.2 Characterization
The crystal structures of the samples were determined by X-ray powder polycrystalline diffractometer (Rigaku D/max-2550VB), using graphite monochromatized Cu Kα radiation (λ = 0.154 nm), operating at 40 kV and 50 mA. The XRD patterns were obtained in the range of 10–80° (2θ) at a scanning rate of 5° min−1. The samples were characterized on a scanning electron microscope (SEM, Hitachi SU-1510) with an acceleration voltage of 15 keV. The samples were coated with 5 nm-thick gold layer before observations. The fine surface structures of the samples were determined by high-resolution transmission electron microscopy (HRTEM, JEOL JEM-2100F) equipped with an electron diffraction attachment with an acceleration voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) measurements were done on a VG ESCALAB MKII XPS system with Mg Kα source and a charge neutralizer. All the binding energies were referenced to the C 1s peak at 284.8 eV of surface adventitious carbon. UV-vis diffused reflectance spectra of the samples were obtained using a UV-vis spectrophotometer (UV-2550, Shimadzu, Japan). BaSO4 was used as a reflectance standard in a UV-vis diffuse reflectance experiment. The photoluminescence (PL) spectra were obtained on Cary Eclipse fluorescence spectrophotometer at room temperature. The PL lifetime was measured using time-resolved fluorescence decay spectra by time-correlated single-photon counting under the 260 nm laser excitation (Cary Eclipse, Agilent). Nitrogen sorption isotherms were performed at 77 K and <10−4 bar on a Micromeritics ASAP2010 gas adsorption analyzer. Each sample was degassed at 150 °C for 5 h before measurements. Surface area was calculated by the Brunauer–Emmett–Teller (BET).
2.3 Evaluation of photocatalytic activity
The photo catalytic activity of the sample was evaluated by the degradation of rhodamine B (RhB) aqueous solution under UV light (λ ≤ 420 nm), using a 300 W Xe arc lamp (CEL-HXF 300) equipped with an ultraviolet cutoff filter as a light source. The reaction system was placed in a sealed black box with the top opened, and was maintained a distance of 15 cm from the light source. The photocatalysts (100 mg) were dispersed in 200 mL of 10 mg L−1 RhB aqueous solution in a Pyrex beaker at room temperature. Before lighting on, the suspension was continuously stirred for 30 min in the dark to ensure the establishment of an adsorption–desorption equilibrium between the catalysts and RhB solution. During degradation, 3 mL of solution was collected by pipette at an interval of irradiation, and subsequently centrifuged to remove the catalysts. UV-vis absorption spectra were recorded on a Spectrumlab 722sp spectrophotometer to determine the concentration of RhB. The degradation reaction could be expressed by an apparent first-order rate constant (ka), which could be calculated using the following equation:| | |
ln(C0/C) = ka × t, or C = C0 × exp(−ka × t)
| (1) |
where C0 is the initial concentration of RhB solution, and C is the concentration of RhB at t-min irradiation, respectively.
2.4 Theoretical calculation
The simulated results of band structures, total and partial densities of states (DOS) were calculated by density functional theory (DFT) as implemented in the CASTEP. The calculations were carried out using the generalized gradient approximation (GGA) level, and Perdew–Burke–Ernzerhof (PBE) formalism for combination of exchange and correlation function. The cut-off energy is chosen as 380 eV, and a density of (3 × 2 × 5) Monkhorst–Pack K-point were adopted to sample the Brillouin zone.
2.5 Electrode fabrication and electrochemistry test
The working electrodes were fabricated by mixing 80 wt% Bi(C2O4)OH, 10 wt% acetylene black, 10 wt% poly(vinylidene fluoride). The mixture was dispersed in 1-methyl-2-pyrrolidinone to form homogeneous slurry under stirring. The slurry was dotted on the surface of a nickel foam electrode and then dried for 24 h at room temperature.
All the electrochemical measurements were carried out on an electrochemical working station (CHI 660D). Electrochemical measurements were carried out in a three-electrode-type cell at room temperature. In the test, a platinum wire served as a counter electrode, a saturated calomel electrode (SCE, Hg/Hg2Cl2) electrode was employed as a reference electrode, and 1 M Na2SO4 aqueous solution was used as the electrolyte. CV and CP were conducted in a potential range of 0.1–0.9 V (versus SCE). Electrochemical impedance spectroscopy (EIS) was performed from 0.1 Hz to 100 KHz at an open circuit potential of 0.8 V and an alternating current (AC) voltage amplitude of 5 mV. The data were analyzed by ZSimWin software.
3. Results and discussion
3.1 Crystal structure and optical properties of Bi(C2O4)OH
Fig. 1a shows the crystal structure of Bi(C2O4)OH, which has the monoclinic space group Pnma with the unit cell parameters of a = 6.0853 (2) Å, b = 11.4479 (3) Å and c = 5.9722 (2) Å.27 It is constructed by BiO6 polyhetra and C2O4 groups. In this structure, two neighboring BiO6 polyhetra connect each other through edge sharing to form a Bi2O2 chain (Fig. 1b), as known from the net structure of [Bi2O2]2+ in Aurivillius-phase (e.g., Bi2MoO6,28 Bi2WO6,29 Bi2SiO5 (ref. 30)) or Sillen-phase (e.g., BiOX, X = Cl, Br, and I).31 These compounds with Aurivillius-phase and Sillen-phase structures show more excellent photooxidation ability and interesting structure–property relationships because of the existence of an active (Bi2O2)2+ layer.
 |
| | Fig. 1 Crystal structure (a), one-dimensional [Bi2O2] chain (b) and UV-vis diffuse reflectance spectra (c) (the inset of absorption1/2 vs. energy in the absorption edge region) of Bi(C2O4)OH (S1-1). | |
Fig. 1c shows the UV-vis diffuse reflectance absorption spectra (DRS) of the as-prepared Bi(C2O4)OH nanorods (S1-2). In the absorption edge region, the square of absorption coefficient is linear with energy for a direct optical transition semiconductor; otherwise the square root of absorption coefficient is linear with energy for an indirect transition semiconductor.32 Observed from Fig. 1c, the optical absorption edge of Bi(C2O4)OH is estimated to be 323 nm and the square root of absorption coefficient is linear with energy. This suggests that the absorption edge of Bi(C2O4)OH is caused by an optical indirect transition.
Band gap of Bi(C2O4)OH is determined by optical absorption near the band edge by the following equation:
where
α,
hν,
A, and
Eg are the optical absorption coefficient, photonic energy, proportionality constant, and band gap, respectivvely.
33 Herein, the
n value is decided by the optical transition type (
n = 1, optical direct absorption;
n = 4, optical indirect absorption). Herein the
n value is 4 for Bi(C
2O
4)OH. As shown in the inset of
Fig. 1c, the
Eg value of Bi(C
2O
4)OH can be estimated to be 3.83 eV by extrapolating the straight line to the
x-axis in this plot. Moreover, we can calculate the conduction and valence bands positions (CB, VB) through the
eqn (3) and
(4) as follows.
where
χ is the absolute electronegativity of semiconductors, which is defined as the geometric average of absolute electronegativity of constituent atoms,
Ee is the energy of free electrons on the hydrogen scale (≈4.5 eV), and
Eg is the band gap.
34 The
χ of Bi(C
2O
4)OH is calculated to be 6.84 eV, and
ECB and
EVB are estimated to be 0.43 and 4.26 eV, respectively.
3.2 Energy band and electronic structures by DFT calculation
The electronic and energy band structures of semiconductor have an important effect on the photoexcitation of charge carriers. Herein, the ab initio density functional theory (DFT) calculations have been carried out to calculate the electronic structures of Bi(C2O4)OH. The band gaps from DFT calculations are usually underestimated, but they often can provide important insight into the physicochemical behavior of semiconductor. As shown in Fig. 2, the valence band maximum (VBM) is located at the X point and conduction band minimum (CBM) is located at the point between G and Z, also demonstrating the fact that Bi(C2O4)OH is an optical indirect band gap semiconductor, in good agreement with the experimental result. The theoretical band gap of Bi(C2O4)OH is 3.01 eV, which is smaller than the experimental value of 3.83 eV due to the underestimation by density function calculation. The total density of states (TDOS) and partial density of states (PDOS) are employed to investigate the electronic properties of Bi(C2O4)OH. The VB top of Bi(C2O4)OH are composed of O 2p orbital and a little of Bi 6p orbital; while the CB bottom is composed of O 2p, C 2p and a little of Bi 6p orbital.
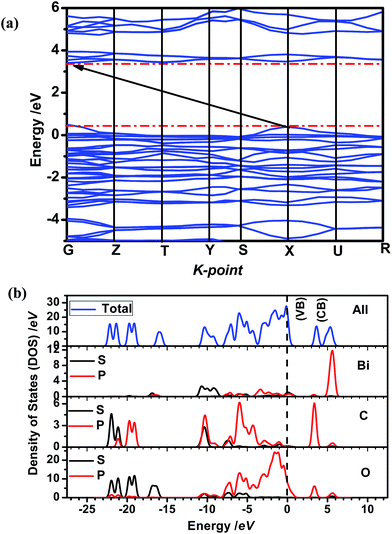 |
| | Fig. 2 (a) Energy band structures and (b) total and partial density of state (TDOS and PDSO) of Bi(C2O4)OH: vertical dash line, valence band maximum (VBM). | |
3.3 Morphology control of Bi(C2O4)OH
In this work, Bi(C2O4)OH was successfully synthesized through a simple one-pot hydrothermal approach. Herein, our particular attention has been paid to the effects of oxalate source, hydrothermal temperature, duration, the volumetric ratio of ethylene glycol (EG) to water (W) and the molar ratio of H2C2O4/Bi(NO3)3 on the products.
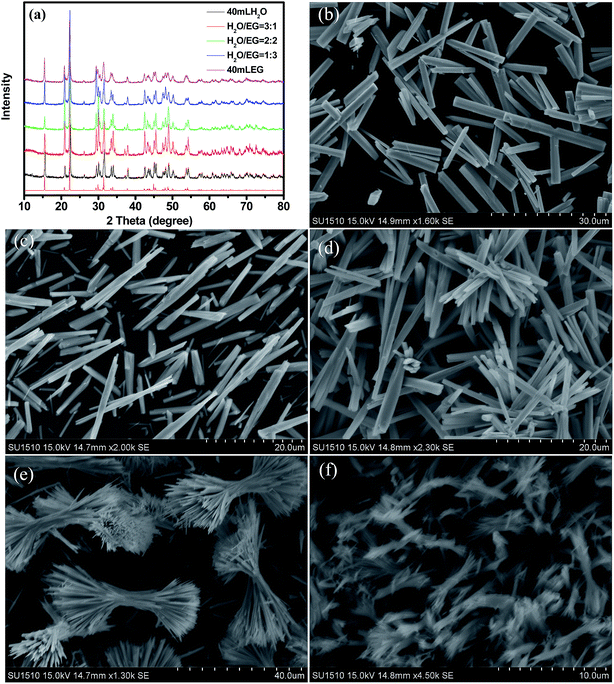
3.3.1. Influence of oxalate source. First, we investigated the influence of oxalate source on the sample (Table 1). These three products with different morphologies (S1-1, S1-2, S1-3) were obtained under the same processing parameters except for different oxalate sources (H2C2O4, (NH4)2C2O4 and Na2C2O4). Fig. 3a gives the typical XRD patterns of the as-synthesized samples. All of the diffraction peaks are in good agreement with the standard card (ISCD#419313). No impurities peaks can be detected in the XRD patterns, indicating the formation of phase-pure Bi(C2O4)OH. When H2C2O4 (pH = 1.6) is used as the oxalate source, the as-obtained sample consists of a large number of uniform microrods, which are 2.3 μm × 22.5 μm large with an aspect ratio of 9.8 (Fig. 3b). When Na2C2O4 (pH = 7.5) is used, the as-obtained sample is composed of nanorods bundles. These nanorods are 400 nm × 4.7 μm large with an aspect ratio of 11.7 (Fig. 3c), which is thinner and shorter than the former. When (NH4)2C2O4 (pH = 6.2) is used, the uniform nanowires are 170 nm × ∼3.5 μm large with an aspect of 20.6 (Fig. 3d). It is obvious that the C2O42− source plays an important role in morphology control of Bi(C2O4)OH. We assume that the strong oxalate source-dependent morphology is closely relative to the pH value of solution. When H2C2O4 is introduced into the solution, the solution can produce Bi3+ and C2O42− immediately under pH = 1.6 condition and therefore nucleation occurs at an outburst speed accompanied by the formation of a large quantity of Bi(C2O4)OH nuclei, which leads to low Bi3+ concentration in solution; that is, the amount of Bi3+ needed to extend the nanostructures is limited. As a result, microrods (Fig. 3b) with 2.3 μm × 22.5 μm large were formed. When (NH4)2C2O4 is added, the number of Bi3+ is much less than those for sample S1-1. The low Bi3+ concentration decreases the rate of Bi(C2O4)OH nuclei, resulting in the increase of aspect ratio than those of sample S1-1. However, when Na2C2O4 is added, the pH value of the solution is 7.5. The very low Bi3+ and C2O42− concentrations in the solution have an effect on the rate of Bi(C2O4)OH nuclei, resulting in the decrease of the diameter and length of the nanorods. It should be noted that all the hydrothermally synthesized different Bi(C2O4)OH morphologies with simply by changing the pH values of the solution.
Table 1 Effect of oxalate source on particle shape and crystal structure of the sample
| Sample |
EG/Wa |
C2O42−/Bib |
C2O42− source |
T/t (°C/h) |
Crystalline phases |
Particle shapec |
| Volumetric ratio of ethylene glycol/deionized water. Molar ratio. Observed by SEM. |
| S1-1 |
0/4 |
3/2 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Microrods |
| S1-2 |
0/4 |
3/2 |
Na2C2O4 |
120/12 |
Bi(C2O4)OH |
Nanorods |
| S1-3 |
0/4 |
3/2 |
(NH4)2C2O4 |
120/12 |
Bi(C2O4)OH |
Nanorods |
 |
| | Fig. 3 XRD patterns (a) and SEM images (b–d) of the samples prepared at 120 °C for 12 h using different oxalate sources: (b) H2C2O4; (c) Na2C2O4; (d) (NH4)2C2O4. | |
3.3.2. Influences of hydrothermal temperature and duration. We have also investigated the influence of hydrothermal temperature on the sample (Table 2 S2-1 to S2-5). The hydrothermal temperature is varied from 100 to 120, 140, 160 and 180 °C, while the other processing parameters are maintained the same. Fig. 4a gives the typical XRD patterns of the as-synthesized samples. At the temperatures lower than 160 °C, the diffraction peaks of all the samples (S2-1, S2-2, S2-3 and S2-4 in Fig. 4a) can be well indexed to single-phase Bi(C2O4)OH (ISCD#419313). At 180 °C, the diffraction peaks of Bi(C2O4)OH, as well as unknown impurities phases, can be observed (Fig. 4a). These results show that the reaction temperature has a significant influence on crystal growth. The morphologies of as-prepared samples were characterized by SEM. At a low reaction temperature (100 °C), S2-1 is composed of nanowires with an average diameter of 200 nm and the length of about 20.5 μm (Fig. 4b). It is interesting that at 120 °C, the uniform straw sheaves (S2-2) have been obtained (Fig. 4c). The individual straw sheaf consists of 400 nm × 35–50 μm nanorods. This novel hierarchical structure of Bi(C2O4)OH has not been reported previously. The straw sheaves have further been investigated by HRTEM (Fig. 4d and e). The nanorod bundles are further confirmed (Fig. 4d). The clear diffraction ring (the inset of Fig. 4d) reveals the polycrystalline nature of Bi(C2O4)OH. Fig. 4e shows the lattice fringes images of the Bi(C2O4)OH straw sheaves. At 140 °C, the sample (S2-3) is exclusively composed of 350 nm × 13 μm nanorods (Fig. 4f), compared with S2-2. At 160 °C, the Bi(C2O4)OH nanorods with an average diameter of around 325 nm and a length of around 12 μm are obtained (Fig. 4g and S2-4). At 180 °C, the 300 nm × 10 μm nanorods, as well as a few of nanoparticles, can be observed (Fig. 4h). It is obvious that the hydrothermal temperature has an important influence on the morphology and structure of the samples. It could be concluded that the nucleation and growth of crystals exist a critical temperature. It is clear that the hydrothermal temperature plays an important role in the formation of high-crystallinity Bi(C2O4)OH. In the latter experiments, 120 °C is selected as the appropriate hydrothermal temperature to synthesize Bi(C2O4)OH straw sheaves.
Table 2 Effects of hydrothermal temperature and duration on particle shape, crystal structure of the sample
| Sample |
EG/W |
C2O4/Bi |
C2O4 source |
T/t (°C/h) |
Crystalline phases |
Particle shape |
| S2-1 |
3/1 |
3/2 |
H2C2O4 |
100/12 |
Bi(C2O4)OH |
Nanorods |
| S2-2 |
3/1 |
3/2 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Straw sheaves |
| S2-3 |
3/1 |
3/2 |
H2C2O4 |
140/12 |
Bi(C2O4)OH |
Nanorods |
| S2-4 |
3/1 |
3/2 |
H2C2O4 |
160/12 |
Bi(C2O4)OH |
Nanorods |
| S2-5 |
3/1 |
3/2 |
H2C2O4 |
180/12 |
Bi(C2O4)OH |
Nanorods |
| S2-6 |
3/1 |
3/2 |
H2C2O4 |
120/3 |
Bi(C2O4)OH |
Nanorods |
| S2-7 |
3/1 |
3/2 |
H2C2O4 |
120/6 |
Bi(C2O4)OH |
Nanorods |
| S2-8 |
3/1 |
3/2 |
H2C2O4 |
120/24 |
Bi(C2O4)OH |
Nanorods |
 |
| | Fig. 4 (a) XRD patterns, (b and c, f–h) SEM and (d and e) HRTEM images (the inset of ED patterns) of the as-prepared samples at different hydrothermal temperatures, corresponding to those in Table 2: (b) 100 °C (S2-1); (c–e) 120 °C (S2-2); (f) 140 °C (S2-3); (g) 160 °C (S2-4); (h) 180 °C (S2-5). | |
Furthermore, Fig. 5a shows the typical X-ray photoelectron spectroscopy (XPS) survey spectrum of Bi(C2O4)OH straw sheaves. The binding energies obtained in the XPS analysis were corrected for specimen charging by referencing C 1s to 284.6 eV. It shows that Bi, O and C elements are contained in Bi(C2O4)OH straw sheaves. Fig. 5b displays the high-resolution XPS spectrum of the Bi 4f. The peaks at about 159.03 eV and 164.34 eV are for the Bi 4f7/2 and Bi 4f5/2, respectively. The result confirms the characteristic Bi3+ in Bi(C2O4)OH. Fig. 5c shows the C 1s spectra of Bi(C2O4)OH. The peak centered at 288.66 eV corresponds to C 1s resultant from C2O42− in Bi(C2O4)OH.35 As shown in Fig. 5d, the O 1s spectrum can be fitted into the peak at 531.4 eV, which can be attributed to the Bi–O bonds of [Bi2O2] slabs.36 Consequently, the phase-pure Bi(C2O4)OH has been obtained.
 |
| | Fig. 5 XPS spectra of Bi(C2O4)OH straw sheaves: (a) survey spectrum; (b) Bi 4f; (c) C 1s; (d) O 1s. | |
To investigate the effect of reaction time, the hydrothermal time is varied from 3 to 6, 12 and 24 h while the hydrothermal temperature is kept at 120 °C (Table 2, S2-2, and S2-6 to S2-8). Fig. 6a shows XRD patterns of the as-prepared samples. The diffraction peaks of all the samples can be well indexed to single-phase Bi(C2O4)OH (ISCD#419313). Fig. 6b shows that the sample (S2-6) is composed of 250 nm × 8 μm nanorods. At 6 h, the as-obtained nanorods (S2-7) turned out to be 14 μm in length and 350 nm in diameter (Fig. 6c). At 12 h, the as-obtained sample consists of uniform straw sheaves, which consist of 0.4 μm × 35–50 μm nanorods (Fig. 6d). At 24 h, the sample (S2-8) is composed of an average diameter of 400 nm and a length of 9 μm (Fig. 6e). The time-dependent experiments suggest that under hydrothermal conditions, the nuclei grow into nanorods, and then the nanorods organize into the hierarchical nanostructures through a self-assembly process. An extensive research is still needed to clarify the details of self-assembly in future.
 |
| | Fig. 6 XRD patterns (a) and SEM images (b–e) of the as-prepared samples at different reaction times, corresponding to those in Table 2: (b) t = 3 h (S2-6); (c) t = 6 h (S2-7); (d) t = 12 h (S2-2); (e) t = 24 h (S2-8). | |
3.3.3. Influences of EG/W volumetric ratio and H2C2O4/Bi(NO3)3 molar ratio. In order to investigate the effect of reaction medium, EG/W volumetric ratio was varied at 0/4, 1/3, 2/2, 3/1 and 4/0 while keeping the other preparations parameters constant (Table 3). Their typical XRD patterns and SEM images are shown in Fig. 7. Fig. 7a shows that the diffraction peaks of all the samples (S1-1, S3-2, S3-3, S2-2 and S3-4 in Table 3) can be well indexed to single-phase Bi(C2O4)OH (ISCD#419313). At EG/W = 0/4 (volumetric ratio), a large number of uniform microrods are obtained, which are around 2.3 μm × 22.5 μm large, with an aspect ratio of 9.8 (Fig. 7b). When the volumetric ratio of EG/W is increased to 1/3, numbers of microrods are obtained, which are about 2.5 μm × 17 μm large, with an aspect ratio of 6.8 (Fig. 7c). At EG/W = 2/2 (volumetric ratio), the 1.6 μm × 13 μm microrods are obtained, with an aspect ratio of 8.1 (Fig. 7d). At EG/W = 3/1, the interesting straw sheaves are achieved, which are assembled by the 400 nm × (30–50) μm nanorods, with the aspect ratios of 37–125 (Fig. 7e). Fig. 7f shows at EG/W = 4/0, a large number of 200 nm × 5 μm nanorods are obtained, with an aspect ratio of 25. These facts indicate that the straw sheaves can only be obtained in the approximate volume ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the EG/W. In other words, EG plays an important role in the formation of straw sheaf hierarchical structure. It is also obvious that the amounts of EG in the solution significantly affect the shape and size of the as-synthesized samples. It is well known that the viscosity of EG (η = 21 mPa s, 20 °C) is much higher than that of water (η = 1.0087 × 10−3 mPa s, 20 °C). The mobility or reactivity of ions in solvent has an important influence on the crystal growth. At an appropriate content of EG (i.e. 3/1 EG/W volumetric ratio), the Bi(C2O4)OH nanorods may stack one another for self-assembly process and favor to form the Bi(C2O4)OH straw sheaves. Although the factor can explain our experimental results well, further investigations are still needed to confirm our hypothesis and reveal the fundamental physical and chemical interactions in our process. To understand the effect of molar ratio of H2C2O4/Bi(NO3)3, the molar ratio of H2C2O4/Bi(NO3)3 was varied at 1/3, 2/3, 1/1, 3/2 and 3/1 while keeping the other preparations parameters constant (Table 4). Fig. 8a confirms the formation of phase-pure Bi(C2O4)OH at different molar ratio of H2C2O4/Bi(NO3)3. The SEM images of the samples are shown in Fig. 8. When the molar ratio of H2C2O4/Bi(NO3)3 is 3/1, the as-obtained sample contains of microrods with an average diameter of around 1 μm and a length of around 35 μm (Fig. 8b). At H2C2O4/Bi(NO3)3 = 3/2 (molar ratio), a number of well-defined straw sheaves are prepared (Fig. 8c). When the molar ratio of H2C2O4/Bi(NO3)3 is increased to 1/1, the larger dumbbells of 22 μm in length are obtained, consisting of 3–4 μm long microrods (Fig. 8d). When the molar ratio of H2C2O4/Bi(NO3)3 is further increased to 2/3, the dumbbells are composed of nanorods with an average diameter of around 500 nm and a length of around 20 μm, some of particles have formed on the dumbbells surfaces (Fig. 8e). At 1/3, Fig. 8f shows that the morphology is made up of some of 15 μm dumbbells long and some spheres with a diameter of 800 nm. These results clearly establish that the H2C2O4/Bi(NO3)3 molar ratio plays a important role in the self-assembly process. Additionally, the amount of Bi(NO3)3 is a crucial factor for the control of the hierarchical structures.
1) of the EG/W. In other words, EG plays an important role in the formation of straw sheaf hierarchical structure. It is also obvious that the amounts of EG in the solution significantly affect the shape and size of the as-synthesized samples. It is well known that the viscosity of EG (η = 21 mPa s, 20 °C) is much higher than that of water (η = 1.0087 × 10−3 mPa s, 20 °C). The mobility or reactivity of ions in solvent has an important influence on the crystal growth. At an appropriate content of EG (i.e. 3/1 EG/W volumetric ratio), the Bi(C2O4)OH nanorods may stack one another for self-assembly process and favor to form the Bi(C2O4)OH straw sheaves. Although the factor can explain our experimental results well, further investigations are still needed to confirm our hypothesis and reveal the fundamental physical and chemical interactions in our process. To understand the effect of molar ratio of H2C2O4/Bi(NO3)3, the molar ratio of H2C2O4/Bi(NO3)3 was varied at 1/3, 2/3, 1/1, 3/2 and 3/1 while keeping the other preparations parameters constant (Table 4). Fig. 8a confirms the formation of phase-pure Bi(C2O4)OH at different molar ratio of H2C2O4/Bi(NO3)3. The SEM images of the samples are shown in Fig. 8. When the molar ratio of H2C2O4/Bi(NO3)3 is 3/1, the as-obtained sample contains of microrods with an average diameter of around 1 μm and a length of around 35 μm (Fig. 8b). At H2C2O4/Bi(NO3)3 = 3/2 (molar ratio), a number of well-defined straw sheaves are prepared (Fig. 8c). When the molar ratio of H2C2O4/Bi(NO3)3 is increased to 1/1, the larger dumbbells of 22 μm in length are obtained, consisting of 3–4 μm long microrods (Fig. 8d). When the molar ratio of H2C2O4/Bi(NO3)3 is further increased to 2/3, the dumbbells are composed of nanorods with an average diameter of around 500 nm and a length of around 20 μm, some of particles have formed on the dumbbells surfaces (Fig. 8e). At 1/3, Fig. 8f shows that the morphology is made up of some of 15 μm dumbbells long and some spheres with a diameter of 800 nm. These results clearly establish that the H2C2O4/Bi(NO3)3 molar ratio plays a important role in the self-assembly process. Additionally, the amount of Bi(NO3)3 is a crucial factor for the control of the hierarchical structures.
Table 3 Effect of EG/W volumetric ratio on particle shape and crystal structure of the sample
| Sample |
EG/W |
C2O4/Bi molar ratio |
C2O4 source |
T/t (°C/h) |
Crystalline phases |
Particle shape |
| S1-1 |
0/4 |
3/2 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Microrods |
| S3-2 |
1/3 |
3/2 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Microrods |
| S3-3 |
2/2 |
3/2 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Microrods |
| S2-2 |
3/1 |
3/2 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Straw sheaves |
| S3-5 |
4/0 |
3/2 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Nanorods |
 |
| | Fig. 7 XRD patterns (a) and SEM images (b–f) of the as-prepared samples at different volumetric ratios of EG/W, corresponding to those in Table 3: (b) 0/4 (S1-1); (c) 1/3 (S3-2); (d) 2/2 (S3-3); (e) 3/1 (S2-2); (f) 4/0 (S3-4). | |
Table 4 Effect of Bi(NO3)3·5H2O amount added on particle shape and crystal structure of the sample
| Sample |
EG/W |
C2O4/Bi molar ratio |
C2O4 source |
T/t (°C/h) |
Crystalline phases |
Particle shape |
| S4-1 |
3/1 |
3/1 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Microrods |
| S2-2 |
3/1 |
3/2 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Straw sheaves |
| S4-3 |
3/1 |
1/1 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Dumbbells |
| S4-4 |
3/1 |
2/3 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Dumbbells + particles |
| S4-5 |
3/1 |
1/3 |
H2C2O4 |
120/12 |
Bi(C2O4)OH |
Dumbbells + spheres |
 |
| | Fig. 8 XRD patterns (a) and SEM images (b–f) of the as-prepared samples at different molar ratios of H2C2O4/Bi(NO3)3, corresponding to those in Table 4: (b) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S4-1); (c) 3 1 (S4-1); (c) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (S2-2); (d) 1 2 (S2-2); (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S4-3); (e) 2 1 (S4-3); (e) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (S4-4); (f) 1 3 (S4-4); (f) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (S4-5). 3 (S4-5). | |
3.3.4. Formation mechanism. Based on the results above, a plausible growth mechanism of straw sheaf is proposed, as shown in Scheme 1. We can conclude that the EG/W mixture medium is essential for the formation of straw sheaf. The time-dependent experiments suggest the following evolution process: at first, EG, as an aprotic and polar solvent can strongly solvate Bi3+ ions. A [Bi(EG)n]3+ complex may form by the coordination bonds between the empty orbits of Bi3+ and lone electron pair of O in EG. After H2C2O4 is added, C2O42− would react with Bi3+ to form Bi(C2O4)OH nuclei and further grow into nanoparticles, as shown in step 1 in Scheme 1. It could be assumed that EG molecules may selectively adsorb on some specific facets of Bi(C2O4)OH (Scheme 1B) to suppress their 2D or 3D growth.37 Then, Bi(C2O4)OH nanorods (Scheme 1D) are produced from these nanoparticles in the latter hydrothermal process (step 2 in Scheme 1).
 |
| | Scheme 1 Formation mechanism of Bi(C2O4)OH straw sheaves. | |
Bi(C2O4)OH is composed of the alternating positive (Bi2O2)n2+ layers and negative C2O42− layers, stacking along the c axis (Scheme 1C). The unique layered structure may favors for the formation of nanorods, the existence of polar charges on their top and bottom surfaces (Scheme 1D). These polar nanorods may connect to each other because of electrostatic effects of these polar charges (Scheme 1E).15 Subsequently, the hierarchical nanostructures (Scheme 1F) can form by stacking. As a result, the nanorods are stacked with each other by their self-assembly process to form hierarchical structure (Scheme 1G). Note that this hypothetic formation mechanism still needs to be confirmed by direct proof in future. Summarily, the reported approach demonstrates the obvious advantages, such as one-step synthesis, environmental friendliness, and low cost.
3.4 Morphology-dependent photoelectrochemistry properties
3.4.1. Photocatalytic performances of Bi(C2O4)OH. To investigate the photocatalytic activity of Bi(C2O4)OH samples for the degradation of organic pollutants, rhodamine B (RhB) are selected as a model pollutant. Its characteristic absorption at about 553 nm is used to monitor the photocatalytic degradation process.38 In the dark, the absorbance at 553 nm of RhB decreased a little in the presence of Bi(C2O4)OH. Table 5 shows Brunauer–Emmett–Teller (BET) surface areas and adsorption percentages of RhB on photocatalysts in the dark after 30 min. The nanorods (S1-2) sample has the largest adsorption capacity for RhB among four samples due to its highest BET area of 6.445 m2 g−1. It clearly revealed that the large BET area of photocatalyst favors for the adsorption of RhB molecules.
Table 5 BET surface areas of Bi(C2O4)OH samples and RhB adsorption percentages
| Sample |
S1-1 |
S1-2 |
S1-3 |
S2-2 |
| BET area [m2 g−1] |
1.232 |
6.445 |
5.756 |
3.618 |
| Adsorption [%] |
2.8 |
9.2 |
8.3 |
5.6 |
Fig. 9a and b shows the degradation curves and reaction kinetic curves of RhB over Bi(C2O4)OH samples under ultraviolet light irradiation (λ ≤ 420 nm). The nanorods (S1-2) sample exhibits a higher photocatalytic activity than the others. In Fig. 9a, RhB has almost be decomposed by the nanorods (S1-2) sample after 120 min, and after 130 min, the degradation efficiencies of RhB over the microrods (S1-1), nanowires (S1-3) and straw sheaves (S2-2) are 30%, 62% and 80%, respectively. The photocatalytic degradation of RhB can be fitted to pseudo first-order kinetics (Fig. 9b). The apparent reaction rate constants (k) are 0.0053, 0.01298, 0.00726, and 0.00888 min−1 for microrods, nanorods, nanowires and straw sheaves, respectively. It is exciting that the nanorods show a photocatalytic activity 2.45, 1.79 and 1.46 times higher than microrods, nanowires and straw sheaves. Since the BET area (6.445 m2 g−1) (Table 5) of nanorods is biggest among the four samples, we hold that BET area mainly contributes to the improved activity of Bi(C2O4)OH nanorods. In addition, the activity of the straw sheaves sample is 1.2 times higher than that of the nanowires sample. Because the BET area (3.618 m2 g−1) of the straw sheaves sample is smaller than that (5.756 m2 g−1) of the nanowires sample, the reflection and absorption of light structure may play key roles in the photocatalytic activity.39 We also have done the UV-vis diffuse reflectance absorption spectra (DRS) analysis to evaluate the impact of photoabsorption of Bi(C2O4)OH samples with different morphologies on the photocatalytic activity, as shown in Fig. S1.† The photoabsorption of all the samples was similar and was located at ∼323 nm. But the intensities of photoabsorption are different. From Fig. S1,† the microrods and nanowires samples show obvious lower intensities than the nanorods and straw sheaves samples. The result is in accordance with the photocatalytic activity. But the photoabsorption intensity of straw sheaves sample is higher than that of nanorods sample, the result is not in accordance with the photocatalytic activity, suggesting that the BET area mainly contributes to the improved activity of Bi(C2O4)OH nanorods. It should be noted that the photoabsorption of Bi(C2O4)OH samples with different morphologies has an impact on the photocatalytic activity. The photoluminescence (PL) spectra of Bi(C2O4)OH samples are shown in Fig. 9c. It is well known that the PL spectra can reflect the recombination rate of photo-generated electron–hole pairs. It is obviously seen that the main emission peak is at 425 nm. The separation efficiency of the photogenerated electrons and holes can be investigated by PL.40 Generally, a low PL emission intensity indicates a low recombination efficiency of photogenerated charges, and thus a high photocatalytic activity. From Fig. 9c, the nanorods sample shows an obvious lower emission than those of microrods, nanowires and straw sheaves, this indicates that the electron–hole pair recombination rate of the nanorods sample is smaller than those of others. Moreover, electrochemical impedance spectroscopy (EIS) is measured to investigate the electron transfer rate (Fig. 9d). In the high frequency region of EIS Nyquist plot, the semicircle radius of the nanorods sample is smaller than those of microrods, nanowires and straw sheaves. A smaller semicircle radius in EIS Nyquist plot means a smaller electric resistance of electrode. It is well-known that the separation efficiency of photoexcited electron–hole pairs is a crucial factor for the photo catalytic activity. A high conductivity of the nanorods sample also favors for electron transportation, thus leading to an efficient charge separation. The result is in accordance with that from PL spectra. This is also in good agreement with their photocatalytic activities.
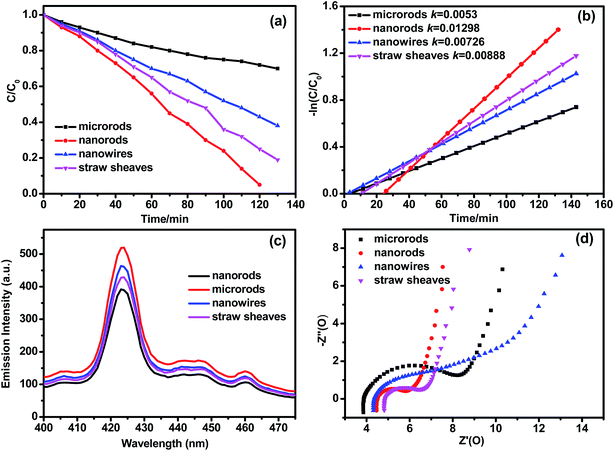 |
| | Fig. 9 (a) Degradation curves and (b) reaction kinetic curves of RhB over Bi(C2O4)OH samples under ultraviolet light irradiation (λ ≤ 420 nm); (c) PL emission spectra and (d) EIS plots. | |
3.4.2. Electrochemistry performances of Bi(C2O4)OH. Layered materials are ideal candidates for electrochemistry.41 To the best of our knowledge, however, the electrochemical performances of anion intercalated bismuth compounds have not been explored yet. Herein, we have investigated the electrochemistry properties of Bi(C2O4)OH as an anion intercalation compound for the first time. The electrochemistry properties of Bi(C2O4)OH samples are investigated by cyclic voltammetry (CV) and chronopotentiometry (CP) in a conventional three-electrode system.The CV measurements of the samples are performed at a scanning rate of 10 mV s−1 in 1 M Na2SO4 aqueous solution (Fig. 10a). The normative rectangular CV curves denote the typical electrical double-layer capacitive behavior, instead of the Faraday reaction. The specific capacitance (C) can be calculated using eqn (5) as follows.
where
C is the discharge capacitance (mF cm
−2),
Q is the charge calculated using the integral area of the CV curve,
S is the real area (cm
−2) of working electrode,
V is the discharge potential (V). The capacitance values of microrods, nanorods, nanowires and straw sheaves samples are 17.29, 19.18, 19.07 and 16.85 mF cm
−2, respectively. It is surprising that the capacitance value of the nanorods sample is 1.11 and 1.14 times higher than those of microrods and straw sheaves samples. Summarily, the nanorods sample shows the largest capacitance of all the samples.
 |
| | Fig. 10 Electrochemistry properties of Bi(C2O4)OH samples: (a) CV curves at different scanning rates; (b) galvanostatic charge–discharge curves at different current densities. | |
Fig. 10b shows the galvanostatic charge–discharge curves of the samples at a current density of 1.0 mA cm−2. The charge/discharge curves indicate a typical electric double-layer capacitive behavior, which is in agreement with the results of the CVs. The discharging capacitance can be calculated according to eqn (6) as follows.
where
C is the discharge capacitance (mF cm
−2),
I is the discharge current (mA),
t is the discharge time (s),
S is the real area (cm
−2) of working electrode,
V is the discharge potential (V). At a current density of 1.0 mA cm
−2, the capacitance values of microrods, nanorods, nanowires and straw sheaves samples are 19.00, 21.00, 20.37 and 17.50 mF cm
−2, respectively. These results are good in agreement with those of the CV measurements.
Generally, EIS is used both to investigate the electrical conductivity and ion transfer of electrochemistry test cells and to study the electrochemical processes. Fig. 9d shows the Nyquist plots of the electrodes in a frequency range from 0.1 Hz to 100 kHz, operated at an open circuit potential of 0.8 V and an alternating current (AC) voltage amplitude of 5 mV. The semicircle is related to the charge transfer resistance (Rctr), which can be evaluated by the diameter of the semicircle. The electrode fabricated by the nanorods sample has the smallest Rctr among the four samples, as indicated by its semicircle (Fig. 9d), the result is in agreement with the CV and CP results. Fig. 9d shows the semicircle of the nanowires is larger than that of microrods. However, the nanowires sample has a larger capacitance than the microrods sample. This demonstrates a higher conductivity for the electrode by the nanowires sample, probably resulting from the BET area. The nanowires sample with a large BET area may endow it with a fast charging rate. The microrods sample shows the largest semicircle among the four samples, but the microrods sample has a larger capacitance than the straw sheaves sample. It may affect by the morphology and the diffusion impedances. Hence, the nanorods sample has a large BET area, making it an excellent electrochemistry material.
4. Conclusions
Multi-scale layered Bi(C2O4)OH compound can be prepared by a simple hydrothermal method. An obvious morphology-dependent photoelectrochemistry property has been revealed. Bi(C2O4)OH nanorods show excellent photocatalytic and electrochemical properties, compare with the other structures (microrods, nanowires and straw sheaves). This work indicates that Bi(C2O4)OH with a featured layer structure can be potentially extended to the other new fields as a multi-functional material.
Acknowledgements
This work is financially supported by National Science Foundation of China (21377060), the Project Funded by the Science and Technology Infrastructure Program of Jiangsu (BM201380277), Jiangsu Science Foundation of China (BK2012862), Six Talent Climax Foundation of Jiangsu (20100292), Jiangsu Province of Academic Scientific Research Industrialization Projects (JHB2012-10), the Key Project of Environmental Protection Program of Jiangsu (2013005), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) sponsored by SRF for ROCS, SEM (2013S002) and “333” Outstanding Youth Scientist Foundation of Jiangsu (2011-2015).
References
- Y. C. Cao, R. Jin and C. A. Mirkin, Science, 2002, 297, 1536–1540 CrossRef CAS PubMed.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS PubMed.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- Y. Zhu, J. Shi, W. Shen, X. Dong, J. Feng, M. Ruan and Y. Li, Angew. Chem., Int. Ed., 2005, 117, 5213–5217 CrossRef.
- F. Favier, E. C. Walter, M. P. Zach, T. Benter and R. M. Penner, Science, 2001, 293, 2227–2231 CrossRef CAS PubMed.
- A. Tao, F. Kim, C. Hess, J. Goldberger, R. He, Y. Xia and P. Yang, Nano Lett., 2003, 3, 1229–1233 CrossRef CAS.
- L. A. Dick, A. D. McFarland, C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2002, 106, 853–860 CrossRef CAS.
- Y. Xiong, J. M. McLellan, J. Chen, Y. Yin, Z. Y. Li and Y. Xia, J. Am. Chem. Soc., 2005, 127, 17118–17127 CrossRef CAS PubMed.
- K. Kim, H. K. Park and N. H. Kim, Langmuir, 2006, 22, 3421–3427 CrossRef CAS PubMed.
- L. Lu, A. Kobayashi, K. Tawa and Y. Ozaki, Chem. Mater., 2006, 18, 4894–4901 CrossRef CAS.
- J. Hu, T. W. Odom and C. M. Lieber, Acc. Chem. Res., 1999, 32, 435–445 CrossRef CAS.
- B. A. Simmons, S. Li, V. T. John, G. L. McPherson, A. Bose, W. Zhou and J. He, Nano Lett., 2002, 2, 263–268 CrossRef CAS.
- C. Ye, G. Meng, Z. Jiang, Y. Wang, G. Wang and L. Zhang, J. Am. Chem. Soc., 2002, 124, 15180–15181 CrossRef CAS PubMed.
- J. Wu, F. Duan, Y. Zheng and Y. Xie, J. Phys. Chem. C, 2007, 111, 12866–12871 CrossRef CAS.
- L. S. Zhang, W. Z. Wang, L. Zhou and H. L. Xu, Small, 2007, 9, 1618–1625 CrossRef PubMed.
- F. Teng, S. Santhanagopalan, A. Asthana, X. Geng, S. I. Mho, R. Shahbazian-Yassar and D. D. Meng, J. Cryst. Growth, 2010, 312, 3493–3502 CrossRef CAS.
- R. Jin, Y. Cao, C. A. Mirkin, K. Kelly, G. C. Schatz and J. Zheng, Science, 2001, 294, 1901–1903 CrossRef CAS PubMed.
- K. S. Lee and M. A. El-Sayed, J. Phys. Chem. B, 2005, 109, 20331–20338 CrossRef CAS PubMed.
- J. Yu and A. Kudo, Adv. Funct. Mater., 2006, 16, 2163–2169 CrossRef CAS.
- J. Wang, F. Teng, M. D. Chen, J. Xu, Y. Song and X. Zhou, CrystEngComm, 2013, 15, 39–42 RSC.
- M. Li, M. D. Chen, J. Wang and F. Teng, CrystEngComm, 2014, 16, 1237–1240 RSC.
- Y. X. Zhao, H. X. Shi, M. D. Chen and F. Teng, CrystEngComm, 2014, 16, 2417–2423 RSC.
- Q. Y. Zhang, H. Tian, N. Li, M. D. Chen and F. Teng, CrystEngComm, 2014, 16, 8334–8339 RSC.
- H. W. Huang, Y. He, X. W. Li, M. Li, C. Zeng, F. Dong, X. Du, T. R. Zhang and Y. H. Zhang, J. Mater. Chem. A, 2015, 3, 24547–24556 RSC.
- H. W. Huang, Y. He, Z. H. Lin, L. Kang and Y. H. Zhang, J. Phys. Chem. C, 2013, 117, 22986–22994 CrossRef CAS.
- K. Xiao, N. Tian, Y. Guo, H. Huang, X. Li and Y. Zhang, Inorg. Chem. Commun., 2015, 52, 5–8 CrossRef CAS.
- M. Rivenet, P. Roussel and F. Abraham, J. Solid State Chem., 2008, 181, 2586–2590 CrossRef CAS.
- X. Zhao, J. Qu, H. Liu and C. Hu, Environ. Sci. Technol., 2007, 41, 6802–6807 CrossRef CAS PubMed.
- C. Zhang and Y. Zhu, Chem. Mater., 2005, 17, 3537–3545 CrossRef CAS.
- R. Chen, J. Bi, L. Wu, W. Wang, Z. Li and X. Fu, Inorg. Chem., 2009, 48, 9072–9076 CrossRef CAS PubMed.
- H. Cheng, B. Huang and Y. Dai, Nanoscale, 2014, 6, 2009–2026 RSC.
- C. Pan and Y. Zhu, Environ. Sci. Technol., 2010, 44, 5570–5574 CrossRef CAS PubMed.
- Y. Ohko, K. Hashimoto and A. Fujishima, J. Phys. Chem. A, 1997, 101, 8057–8062 CrossRef CAS.
- P. Madhusudan, J. Ran, J. Zhang, J. Yu and G. Liu, Appl. Catal., B, 2011, 110, 286–295 CrossRef CAS.
- Q. Li, H. Liu, F. Dong and M. Fu, J. Colloid Interface Sci., 2013, 408, 33–42 CrossRef CAS PubMed.
- F. Dong, S. Lee, Z. Wu, Y. Huang, M. Fu, W. K. Ho, S. Zou and B. Wang, J. Hazard. Mater., 2011, 195, 346–354 CrossRef CAS PubMed.
- J. Geng, D. Lu, J. J. Zhu and H. Y. Chen, J. Phys. Chem. B, 2006, 110, 13777–13785 CrossRef CAS PubMed.
- H. Fu, L. Zhang, W. Yao and Y. Zhu, Appl. Catal., B, 2006, 66, 100–110 CrossRef CAS.
- K. L. Zhang, C. M. Liu, F. Q. Huang, C. Zheng and W. D. Wang, Appl. Catal., B, 2006, 68, 125–129 CrossRef CAS.
- Y. Wang, X. C. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2011, 50, 2–24 CrossRef.
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395–1397 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00917d |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the EG/W. In other words, EG plays an important role in the formation of straw sheaf hierarchical structure. It is also obvious that the amounts of EG in the solution significantly affect the shape and size of the as-synthesized samples. It is well known that the viscosity of EG (η = 21 mPa s, 20 °C) is much higher than that of water (η = 1.0087 × 10−3 mPa s, 20 °C). The mobility or reactivity of ions in solvent has an important influence on the crystal growth. At an appropriate content of EG (i.e. 3/1 EG/W volumetric ratio), the Bi(C2O4)OH nanorods may stack one another for self-assembly process and favor to form the Bi(C2O4)OH straw sheaves. Although the factor can explain our experimental results well, further investigations are still needed to confirm our hypothesis and reveal the fundamental physical and chemical interactions in our process. To understand the effect of molar ratio of H2C2O4/Bi(NO3)3, the molar ratio of H2C2O4/Bi(NO3)3 was varied at 1/3, 2/3, 1/1, 3/2 and 3/1 while keeping the other preparations parameters constant (Table 4). Fig. 8a confirms the formation of phase-pure Bi(C2O4)OH at different molar ratio of H2C2O4/Bi(NO3)3. The SEM images of the samples are shown in Fig. 8. When the molar ratio of H2C2O4/Bi(NO3)3 is 3/1, the as-obtained sample contains of microrods with an average diameter of around 1 μm and a length of around 35 μm (Fig. 8b). At H2C2O4/Bi(NO3)3 = 3/2 (molar ratio), a number of well-defined straw sheaves are prepared (Fig. 8c). When the molar ratio of H2C2O4/Bi(NO3)3 is increased to 1/1, the larger dumbbells of 22 μm in length are obtained, consisting of 3–4 μm long microrods (Fig. 8d). When the molar ratio of H2C2O4/Bi(NO3)3 is further increased to 2/3, the dumbbells are composed of nanorods with an average diameter of around 500 nm and a length of around 20 μm, some of particles have formed on the dumbbells surfaces (Fig. 8e). At 1/3, Fig. 8f shows that the morphology is made up of some of 15 μm dumbbells long and some spheres with a diameter of 800 nm. These results clearly establish that the H2C2O4/Bi(NO3)3 molar ratio plays a important role in the self-assembly process. Additionally, the amount of Bi(NO3)3 is a crucial factor for the control of the hierarchical structures.
1) of the EG/W. In other words, EG plays an important role in the formation of straw sheaf hierarchical structure. It is also obvious that the amounts of EG in the solution significantly affect the shape and size of the as-synthesized samples. It is well known that the viscosity of EG (η = 21 mPa s, 20 °C) is much higher than that of water (η = 1.0087 × 10−3 mPa s, 20 °C). The mobility or reactivity of ions in solvent has an important influence on the crystal growth. At an appropriate content of EG (i.e. 3/1 EG/W volumetric ratio), the Bi(C2O4)OH nanorods may stack one another for self-assembly process and favor to form the Bi(C2O4)OH straw sheaves. Although the factor can explain our experimental results well, further investigations are still needed to confirm our hypothesis and reveal the fundamental physical and chemical interactions in our process. To understand the effect of molar ratio of H2C2O4/Bi(NO3)3, the molar ratio of H2C2O4/Bi(NO3)3 was varied at 1/3, 2/3, 1/1, 3/2 and 3/1 while keeping the other preparations parameters constant (Table 4). Fig. 8a confirms the formation of phase-pure Bi(C2O4)OH at different molar ratio of H2C2O4/Bi(NO3)3. The SEM images of the samples are shown in Fig. 8. When the molar ratio of H2C2O4/Bi(NO3)3 is 3/1, the as-obtained sample contains of microrods with an average diameter of around 1 μm and a length of around 35 μm (Fig. 8b). At H2C2O4/Bi(NO3)3 = 3/2 (molar ratio), a number of well-defined straw sheaves are prepared (Fig. 8c). When the molar ratio of H2C2O4/Bi(NO3)3 is increased to 1/1, the larger dumbbells of 22 μm in length are obtained, consisting of 3–4 μm long microrods (Fig. 8d). When the molar ratio of H2C2O4/Bi(NO3)3 is further increased to 2/3, the dumbbells are composed of nanorods with an average diameter of around 500 nm and a length of around 20 μm, some of particles have formed on the dumbbells surfaces (Fig. 8e). At 1/3, Fig. 8f shows that the morphology is made up of some of 15 μm dumbbells long and some spheres with a diameter of 800 nm. These results clearly establish that the H2C2O4/Bi(NO3)3 molar ratio plays a important role in the self-assembly process. Additionally, the amount of Bi(NO3)3 is a crucial factor for the control of the hierarchical structures.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S4-1); (c) 3
1 (S4-1); (c) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (S2-2); (d) 1
2 (S2-2); (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S4-3); (e) 2
1 (S4-3); (e) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (S4-4); (f) 1
3 (S4-4); (f) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (S4-5).
3 (S4-5).




