An “off–on” fluorescent probe for the detection of cysteine /homocysteine and its imaging in living cells†
Mengfang Tang‡
a,
Luling Wu‡b,
Dan Wua,
Chusen Huang*b,
Weiping Zhu*a,
Yufang Xua and
Xuhong Qiana
aState Key Laboratory of Bioreactor Engineering, Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China. E-mail: wpzhu@ecust.edu.cn
bThe Education Ministry Key Laboratory of Resource Chemistry, Shanghai Key Laboratory of Rare Earth Functional Materials, Department of Chemistry, College of Life and Environmental Sciences, Shanghai Normal University, 100 Guilin Road, Shanghai 200234, China. E-mail: huangcs@shnu.edu.cn
First published on 4th April 2016
Abstract
Based on the NCL reaction, we report a novel “off–on” fluorescent probe BQ for the sensing of Cys/Hcy by a FRET strategy. Owing to the quenching effect of azo-based naphthalimide, BQ showed nonfluorescence. Upon the interaction of Cys/Hcy, a dramatic increase of the fluorescence signal was observed, with a high selectivity toward Cys/Hcy over GSH. Additionally, the discrimination of Cys from Hcy also could be achieved through the different rates of the transthioesterification reaction between thiols and BQ, especially when the concentration of Cys and Hcy was below 100 μM. The Cys/Hcy could be specifically labeled by the BODIPY fluorophore of BQ after the NCL reaction. Living cell imaging experiments demonstrated that BQ can selectively label Cys/Hcy and distinguish Cys/Hcy from other bioreagents including GSH in living systems.
Biological thiols are the essential factors in regulating cellular function through maintaining the microenvironment redox homeostasis.1–3 These small-molecular-weight biothiols including cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) are active oxidative–reductive agents and act as antioxidants protecting against damage from the reactive oxygen species, which is relevant to the growth of cells and tissues in living systems.4,5 Specifically, Cys deficiency is involved in many syndromes, for instance, slow growth in children, muscle and fat loss, edema, liver damage, skin lesions and weakness, as well as HIV infection.6,7 Similarly, the elevated levels of plasma total Hcy (>12 μM) have been considered as an important risk factor for cardiovascular diseases, Alzheimer's disease, neural tube defects, complications during pregnancy, inflammatory bowel disease and osteoporosis.8,9 Meanwhile, markedly increased concentrations of Cys and Hcy have been associated with neurotoxicity.10 Thus, development of efficient tools for determining Cys/Hcy in biological samples, especially for the simultaneous tracing of Cys/Hcy in living cells is fundamental for understanding their physiological and cell biological significance.
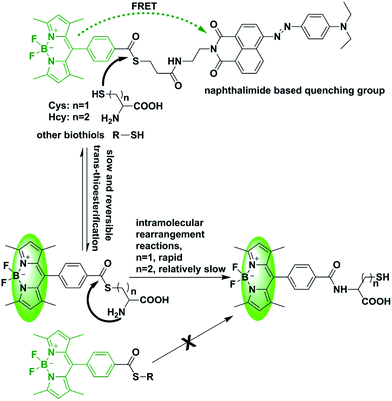
Fluorescent probes, which can image and track intracellular Cys and Hcy, could provide a convenient tool to study the distribution of these biothiols in addition to their concentrations. In recent years, based on different mechanisms, a large number of fluorescent probes for biological thiols have been exploited.11–15 However, the major challenge for detecting Cys and Hcy in biological samples especially in live cells is from the interference of GSH, the high concentration of which (usually 1–10 mM) will also give fluorescence response to the most designed probes. Therefore, designing and synthesizing efficient fluorescent probes for discriminating Cys/Hcy from GSH is still urgently needed. In addition, unlike GSH, Cys is the final product of the transsulfuration pathway through Hcy.16–18 It is of great significance to investigate the activity and metabolic pathways of Cys/Hcy within cells. Hence, to unravel the complicated biomedical mechanisms by which Cys and Hcy are involved in various disease states, effective tools that show different fluorescent signals in response to Cys and Hcy, respectively, are also highly desirable. Native chemical ligation (NCL) technology is by far the most effective, commonly used and in-depth researched method for the polypeptide ligation. NCL involves cascade reactions between a C-terminal thioester peptide and another peptide containing an N-terminal Cys residue.19 In the first step of NCL, there is an ester exchange reaction between foreign thiol and peptide thioester. The reversible transthioesterification reaction between a C-terminal thioester peptide and the sulfhydryl group of an N-terminal Cys residue affords a thioester-linked intermediate. Then thioester-linked intermediate undergoes a spontaneous and rapid intramolecular S–N acyl shift reaction with the adjacent terminal amino of Cys residue, which results in the formation of a stable and native amide bond at the ligation site to connect the polypeptide. Thus NCL could provide a new vision for designing and synthesizing probes for the selective detection of an amino-terminal Cys residue, and some NCL based fluorescent probes have been developed for selective detection of Cys/Hcy.20–22 However, those probes were synthesized by introducing the thiophenol ester as the selective recognition group (Fig. S1†). Despite the sensitivity could be enhanced due to that thiophenol ester favored forming the aliphatic thioester in the presence of aliphatic thiols group through the reversible transthioesterification reaction.23 Other biothiols such as GSH may also give response to these designed probes,20 which will reduce the selectivity of the probes. Herein, we introduced the aliphatic thioester to construct a new NCL based fluorescent probe for the discrimination between Cys and Hcy, as well as GSH (Fig. S1†). Our rationale is depicted in Scheme 1. The probe is composed of a BODIPY dye, an aliphatic thioester group, and an azo-based naphthalimide (an effective quencher moiety). The fluorescence signal from BODIPY dye will be quenched by azo based naphthalimide through the fluorescence resonance energy transfer (FRET) mechanism. According to the NCL reaction, trans-thioesterification of aliphatic thioester will happen, but is a relatively slow and also reversible process.23 We reasoned that the mixture of probe with Cys/Hcy would result in the formation of the corresponding amide via a tandem NCL reaction, which could pull the trans-thioesterification equilibrium leading to the cleavage of the FRET dyad. But for the GSH, intramolecular rearrangement reactions will not occur after the trans-thioesterification, therefore the native amide bond will not form via the tandem NCL reaction between the BODIPY dye and GSH. Thus the designed probe has a unique “Off–On” effect exclusively towards Cys/Hcy, allowing selective detection excluding GSH. The second advantage of this design is that Cys/Hcy could be specifically labelled by the BODIPY fluorophore of BQ after the NCL reaction. Some of the developed fluorescent probes could specifically recognizing Cys/Hcy, however, the fluorophore of the fluorescent probes diffused away after their recognizing the target Cys/Hcy.11–15 Thus the Cys/Hcy could not be specifically labelled and their intracellular dynamic changes might not be clearly captured through the visible fluorescence signal.
To demonstrate the aforementioned hypothesis, we first synthesized the target probe BQ from available starting materials (see Scheme S1 in the ESI†). The chemical structure of BQ was well characterized by 1H NMR, 13C NMR and HRMS.
We anticipated that BQ would have a different fluorescence response towards biothiols with or without an adjacent amino group, Cys/Hcy and GSH, respectively, according to the designed chemical structure of this probe. This hypothesis is verified by spectrographic experiments. Fluorescence response of BQ in the presence of Cys, Hcy or GSH (1 mM) was measured at 25 °C in PBS–CH3CN buffer (10 mM, pH 7.4, 45% acetonitrile) (Fig. S2†). BQ exhibits an intense fluorescence response to Cys at 510 nm, and a relatively low response to Hcy. However, the fluorescence response to GSH is negligible. Even the concentration of GSH increased to 10 mM and 20 mM, respectively, the fluorescence intensity is still low compared to the fluorescence response of Hcy/Cys for BQ in the same test conditions (Fig. S2†). Then the detection mechanism was further investigated by mass spectrometry. The molecular ion peaks of BODIPY-Cys (m/z = 473.3 (M + H+), 495.2 (M + Na+))/BODIPY-Hcy (m/z = 509.2 (M + Na+)) were observed. Meanwhile, the BODIPY-GSH (m/z = 657.3 (M + Na+)) was also observed. But BODIPY-GSH was not the main molecular ion peak, as the relative abundance of BODIPY-GSH was relative lower compared to BODIPY-Cys/BODIPY-Hcy (Fig. S3–S5†). This results suggested that trans-thioesterification of aliphatic thioester will happen, but is a relatively slow and also reversible process. Cys/Hcy could pull the trans-thioesterification equilibrium leading to the cleavage of the FRET dyad due to NCL reaction. But for GSH, intramolecular rearrangement reactions will not occur after the trans-thioesterification, and then the reaction between BQ and GSH was slow and reversible. Furthermore, the molecular ion peaks of azo-based naphthalimide BQ-5 (m/z = 525.2 (M + Na+)) was also observed (Fig. S3–S5†). This demonstrated the successful cleavage of the FRET dyad azo-based naphthalimide in the presence of Cys/Hcy. And the fluorescence increase is from the inhibition of FRET process.
Next, we performed fluorescence titration experiments to evaluate the sensitivity of BQ towards Cys and Hcy. The results are summarized and shown in Fig. 1. Without any substrate, the fluorescence intensity of BQ is very weak, which is attributed to the quenching effect of the azo group as expected. After incubation with Cys for 30 minutes, the emission intensity increased by 100-fold with 1 mM Cys and 230-fold with 3 mM Cys at 510 nm (Fig. 1a). The fluorescence intensity also increased in accordance with the increasing concentrations of Hcy (Fig. 1b). Additionally, through the quantitative analysis, we could clearly observed the gradually enhancement in fluorescence intensity with the elevated Cys/Hcy concentrations. It is notable that the fluorescence enhancement in Cys is higher than the enhancement in Hcy (Fig. 1c), identifying the higher rates of transthioesterification reaction of Cys for BQ compared to Hcy at the same concentration. Thus, BQ could be further used for discriminating Cys from Hcy through the different transthioesterification reaction of Cys/Hcy. Moreover, it is interesting that a linear relationship between changes of fluorescence intensity and the concentration of Cys changes from 0 μM to 300 μM (Fig. 1d) was obtained. While only background signal was obtained when the concentration range of Hcy is 0–100 μM, and then exhibiting a linear relationship between changes of fluorescence intensity and the concentration of Hcys changes from 100 μM to 300 μM (Fig. 1d). The intracellular concentration of Cys is usually in the range of 50–300 μM, and the Hcy is about 5–10 μM within the cells, even the abnormally elevated Hcy in some diseases is less than 50 μM,4,8 which makes BQ potentially application in discriminating intracellular Cys from Hcy.
The time-dependent fluorescence response of BQ in the presence of Cys or Hcy (1 mM) was measured at room temperature in PBS–CH3CN buffer (10 mM, pH 7.4, 45% acetonitrile) (Fig. 2a and b). It was found that the fluorescence intensity at 510 nm increased with the addition of Cys, and reached a plateau after 30 minutes (Fig. 2a and c). Compared with Cys, fluorescence intensity at 510 nm increased slowly with the addition of Hcy, and reached a plateau after 2 hours under the same conditions (Fig. 2b and c). This different reaction kinetics between Cys and Hcy for probe BQ can be attributed to that the amino group of Hcy is away from thiol group (–SH group) compared to the Cys, leading to the relative slow intramolecular rearrangement reaction after the trans-thioesterification of Hcy. The similar phenomena could also be observed when the concentration of Cys/Hcy was 200 μM (Fig. S6†). Thus, by using BQ, we could also discriminate Cys from Hcy through their different fluorescence response to BQ in reaction kinetics (Fig. 2c).
To investigate its selectivity, probe BQ (1 μM) was treated with representative amino acids (such as Cys, Hcy, GSH, Glu, Ser, Met, Thr, His, Leu, Tyr) in PBS–CH3CN buffer and monitored by emission spectroscopy. As exhibited in Fig. 2d, there is a significant increase in the fluorescence intensity, with 100- and 34-fold enhancements at 510 nm for Cys and Hcy, respectively. In contrast, the other amino acids elicited no visible changes in emission. The results demonstrated that probe BQ has a high selectivity for Cys and Hcy.
Finally, BQ was used for detecting Cys/Hcy in live cells. Initially, the cell toxicity of BQ was tested by CCK-8 assay. As shown in Fig. S7,† the cell viability was above 95% when the concentration range of BQ was 0–30 μM. Then, to examine the possibility of BQ for imaging applications in living cells, the live HeLa cells were incubated with BQ (2 μM) at 37 °C for 10 min, following by addition of 1 mM Cys, Hcy and GSH, respectively (the detailed experiments, please see the ESI†). And we could observe a remarkably fluorescence signal from the intracellular zone (Fig. 3, Cys and Hcy channel). However, we could note essentially no fluorescence when the HeLa cells were treated with GSH at the same bio-imaging conditions (Fig. 3, GSH channel). In contrast, when cells were only incubated with BQ, while no Cys or Hcy were loaded into cells, there was only intracellular background fluorescence under the same bio-imaging conditions (Fig. 3, probe channel). The suppressing experiment was also conducted. After the cells were pretreated with N-ethylmaleimide (NEM, a thiol-specific alkylation reagent), and then BQ was added into cells, after incubation for 10 min, the Cys or Hcy was loaded into the live cells. As shown in Fig. S8,† no fluorescence signal could be observed in Cys and Hcy treated cells, respectively, which could be ascribed to that NEM could selectively interact with thiols in Cys/Hcy and then inhibited the interaction of BQ with Cys/Hcy. Thus, the results suggest that BQ can easily penetrate cell membranes and make fast fluorescent labeling for Cys or Hcy in live cells, and the GSH has no interference.
Furthermore, after different concentrations of Cys, Hcy and GSH was added into HeLa cells, respectively, there was different fluorescence response of BQ was observed (Fig. 4). When the concentration was 50 μM, the cells treated with Hcy and GSH have no fluorescence signal. And cells treated with Cys exhibited visible fluorescence signal. After the concentration increased to 200 μM, a remarkable fluorescence signal could be obtained from the cells treated with Cys and Hcy, while there was no fluorescence signal in cells treated with GSH. Even the concentration increased to 10 mM, still no significant fluorescence signal was obtained (Fig. S9†) by compared to the Cys/Hcy treated cells. These results suggested that BQ could discriminate Cys/Hcy from GSH in live cells. Additionally, during the low concentration range, BQ could be used for discriminating Cys from Hcy in live cells, which was consistent with the results in Fig. 1 and 2, and could be ascribed to the different rates of transthioesterification reaction of Cys and Hcy for BQ. Considering the relationship between intracellular Cys and Hcy levels, and their function in many diseases, BQ could provide a visible and efficient way for further investigating the fundamental roles of Cys/Hcy in regulating the cellular redox environments, which will be significant for understanding the Cys/Hcy related diseases in cellular level.
In summary, based on the NCL reaction, we report a novel “off–on” fluorescent probe BQ for the sensing of Cys/Hcy by FRET strategy. Owing to the quenching effect of azo-based naphthalimide, BQ was nonfluorescent. But the reaction of BQ with Cys/Hcy resulted in a dramatic increase of fluorescence signal with a high selectivity toward Cys/Hcy over GSH. Additionally, the discrimination of Cys from Hcy also could be achieved through the different rates of transthioesterification reaction of thiols and BQ, especially when the concentration of Cys and Hcy was below 100 μM. Finally BQ was successfully applied to the direct cellular imaging of Cys/Hcy in living cells. To best of our knowledge, BQ is the rarely developed FRET-based fluorescent probe for determination of Cys/Hcy without GSH interference by introducing the NCL reaction strategy. We anticipate the probe BQ can be served as an efficient tool for further investigation of Cys/Hcy-related pathology.
Acknowledgements
This work was supported by the financial support from National Natural Science Foundation of China (21476077, 21236002, 21302125), Shanghai Pujiang Program and the Fundamental Research Funds for the Central Universities.Notes and references
- J. S. Stamler and A. Slivka, Nutr. Rev., 1996, 54, 1–30 CrossRef CAS PubMed.
- S. Iwata, T. Hori, N. Sato, Y. Ueda-Taniguchi, T. Yamabe, H. Nakamura, H. Masutani and J. Yodoi, J. Immunol., 1994, 152, 5633–5642 CAS.
- H. Nakamura, K. Nakamura and J. Yodoi, Annu. Rev. Immunol., 1997, 15, 351–369 CrossRef CAS PubMed.
- R. Carmel and D. W. Jacobsen, Homocysteine in health and disease, Cambridge University Press, 2001 Search PubMed.
- S. Vertuani, A. Angusti and S. Manfredini, Curr. Pharm. Des., 2004, 10, 1677–1694 CrossRef CAS PubMed.
- S. Shahrokhian, Anal. Chem., 2001, 73, 5972–5978 CrossRef CAS PubMed.
- K. R. Atkuri, J. J. Mantovani, L. A. Herzenberg and L. A. Herzenberg, Curr. Opin. Pharmacol., 2007, 7, 355–359 CrossRef CAS PubMed.
- S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostino, P. W. F. Wilson and P. A. Wolf, N. Engl. J. Med., 2002, 346, 476–483 CrossRef CAS PubMed.
- P. M. Ueland and S. E. Vollset, Clin. Chem., 2004, 50, 1293–1295 Search PubMed.
- J. Klingman and D. Choi, Neurology, 1989, 39, 397–398 Search PubMed.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- X. Yang, Y. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690–10693 CrossRef CAS PubMed.
- H. S. Jung, X. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019–6031 RSC.
- C. Yin, F. Huo, J. Zhang, R. Martinez-Manez, Y. Yang, H. Lv and S. Li, Chem. Soc. Rev., 2013, 42, 6032–6059 RSC.
- H. Peng, W. Chen, Y. Cheng, L. Hakuna, R. Strongin and B. Wang, Sensors, 2012, 12, 15907–15946 CrossRef CAS PubMed.
- W. Wang, O. Rusin, X. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15949–15958 CrossRef CAS PubMed.
- J. M. Williamson, B. Boettcher and A. Meister, Proc. Natl. Acad. Sci. U. S. A., 1982, 79, 6246–6249 CrossRef CAS.
- M. H. Hanigan and W. A. Ricketts, Biochemistry, 1993, 32, 6302–6306 CrossRef CAS PubMed.
- P. Dawson, T. Muir, I. Clark-Lewis and S. Kent, Science, 1994, 266, 776–779 CAS.
- L. Long, W. Lin, B. Chen, W. Gao and L. Yuan, Chem. Commun., 2011, 47, 893–895 RSC.
- L. Yuan, W. Lin, Y. Xie, S. Zhu and S. Zhao, Chem.–Eur. J., 2012, 18, 14520–14526 CrossRef CAS PubMed.
- X.-F. Yang, Q. Huang, Y. Zhong, Z. Li, H. Li, M. Lowry, J. O. Escobedo and R. M. Strongin, Chem. Sci., 2014, 5, 2177–2183 RSC.
- J. Kalia and R. T. Raines, ChemBioChem, 2006, 7, 1375–1383 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00832a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |