In silico study on baicalein and baicalin as inhibitors of dengue virus replication
Abstract
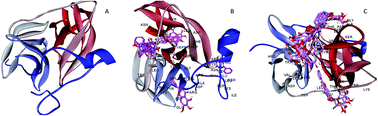
The dengue virus (DENV) is an important human arbovirus that belongs to the Flaviviridae. Currently, there is no vaccine or effective antiviral agent against DENV. Therefore, finding an efficient antiviral agent against this virus is crucial. We have previously reported the anti-DENV activity of two flavonoids, namely, baicalein and baicalin, against different stages of the virus replication cycle in Vero cells. In this study, we aimed to predict the possible interactions between viral proteins that are important for DENV replication and these two flavonoids as potential candidates for anti-DENV drug discovery with known in vitro anti-DENV activity. In this study, the interactions between compounds of interest and three important proteins of DENV were predicted using appropriate software. Moreover, the binding energy between these compounds and selected proteins was calculated as one of the main criteria for molecular docking studies. The results showed that both compounds of interest, as ligands, can bind with chosen viral proteins, as receptors, through hydrogen bonding and other interactions such as pi–pi interactions, pi–sigma interactions and pi–cation interactions. The obtained data showed significant affinity between the tested compounds and the NS2B–NS3 protease of DENV. Therefore, an in vitro anti-protease assay was conducted and the results showed significant anti-DENV protease activity for both compounds, especially baicalein. In conclusion, our obtained data showed that both tested compounds can affect DENV intracellular replication and internalization. However, these results support our previous findings from in vitro studies and encourage us to further studies towards finding the mechanism of action of these compounds.

 Please wait while we load your content...
Please wait while we load your content...