Fabrication of a polyaniline/MoS2 nanocomposite using self-stabilized dispersion polymerization for supercapacitors with high energy density†
Abstract
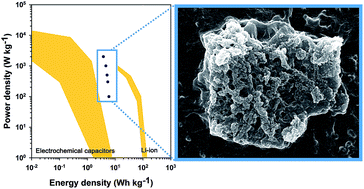
In this report, a polyaniline/MoS2 nanocomposite has been firstly produced using a self-stabilized dispersion polymerization method. The synthesized polyaniline/MoS2 nanocomposite exhibited a remarkably high electrical conductivity of ca. 28.6 S cm−1, which is higher than other previously reported MoS2-based composites. Additionally, the PANI/MoS2 nanocomposite exhibited substantially improved capacitance (ca. 400 F g−1) compared to pristine MoS2 nanosheets (ca. 3 F g−1) and PANI (ca. 232 F g−1) and enhanced cycling stability (retention rate of 84%) in comparison with pure PANI (retention rate of 62%). Furthermore, the PANI/MoS2 nanocomposite demonstrated a higher energy density (4.7 W h kg−1 at 1000 W kg−1) than conventional electrochemical capacitors and other previously reported carbon and carbon/conducting polymer based electrochemical capacitors owing to its high utilizing of pseudocapacitance attributed to high electrical conductivity. What is more, the synthesized PANI/MoS2 nanocomposite demonstrated good rate capability and a good power characteristic as the supercapacitor electrode by keeping its high energy density (3.8 W h kg−1) at a high power density (2000 W kg−1) due to the existence of sufficient empty space between interconnected PANI nanofibers and high electrical conductivity of the PANI/MoS2 nanocomposite.
- This article is part of the themed collection: 2D Materials: Explorations Beyond Graphene

 Please wait while we load your content...
Please wait while we load your content...