A new indicator to evaluate the pollution of iron and manganese
Xu Zhanga,
Huanhuan Yangb and
Zhaojie Cui*a
aSchool of Environmental Science and Engineering, Shandong University, Ji'nan 250100, China. E-mail: cuizj@sdu.edu.cn
bSchool of Life Science, Shandong University, Ji'nan 250100, China
First published on 10th March 2016
Abstract
Heavy metals in Anshan tailings is in line with Chinese national standards, but it still contains a huge amount of iron and manganese, much higher than the soil background level in China. Iron and manganese leaching into water via precipitation can cause water pollution. The serious acid rain in Liaoning Province exacerbates the process of migration and transformation. Based on the rainfall characteristics of Liaoning Province, the study simulated the leaching process of acid rain to explore the release effect of iron and manganese with different pH values. We studied the behavior of Danio rerio to evaluate the contamination of iron and manganese, finally to obtain an online monitoring method for heavy metal pollution in water. SOD and GPX were measured to explore the toxicity mechanism of iron and manganese. Activated sludge, as a kind of cheap, efficient and recyclable material, has a great effect on removing heavy metals in water. This study is the first to use activated sludge to remove iron and manganese in water, proving that the activated sludge has a significant effect on removal of iron and manganese.
1. Introduction
With heavy metal pollution getting worse every day, the related research gets more thorough and comprehensive. Mining, smelting, forging, transportation and other industrial processes will produce large amounts of heavy metals.1 Exhaust emissions, waste water and residues are directly released into the atmosphere, water and soil, so that the concentration of heavy metals seriously exceeds standard levels in the environment.2Composite pollution index, also known as Nemerow index, is a multi-weighted environmental quality index, which is taken extreme value into account.3 Nemerow index specifically is widely used in identifying the most polluted factor and avoiding the influence of subjective factors.
The increasing consumption of fossil fuel has led to the increase of sulfur oxides, and nitrogen oxides, which are eventually transformed into acid deposition.4 Acid rain has an adverse impact on water quality by destroying the ecosystem, affecting plant growth, and altering aquatic organisms community even to death.5 With the development of heavy industry, precipitation acidity in northeastern China has increased year by year. Acid rain in those area is mainly caused by sulfuric acid type, due to a large number of SO2 released by burned coal.6 The acid rain has significant influence on heavy metal pollution in soil because those elements can leach into water through the rain.7 On one hand, it will result in activating heavy metal in soil,8 accumulating them in crops, even affecting agricultural production. On the other hand, massive release of heavy metal ions will cause serious toxic effects in water.9
The accidental pollution events in aquatic environment have been frequently reported.10 An early warning system is required to deal with those events. Biological behavior is external reaction caused by internal environment change in the stimulation of outside environment.11 We can use behavioral response of aquatic organisms to monitor on water quality, and to implement the qualitative and semi-quantitative analysis.12
Based on previous research, activated sludge with high specific surface area has been widely used in removal of heavy metals.13 In this study, activated sludge is firstly applied to absorb iron and manganese in water.
2. Materials and methods
2.1. Study area
Anshan city is located in Northeast of China and is famous for iron and steel industry. The experimental soil was sampled from the Anshan tailings.The experimental Danio rerio was cultured by our laboratory, using the feeding methods of water circulation and the activated carbon and ceramic rings to ensure water quality. Control illumination period and water temperature at 26 ± 2 °C. Feed and clean the aquarium regularly to ensure fish healthy. Length of Danio rerio was about 3.0 cm and weight about 0.3 g.
Activated sludge was supplied by Jinan Everbright water Ltd, which is a large-scale sewage treatment enterprise, mainly focuses on sewage treatment and sludge exploitation.
2.2. Sampling and detection
We set up three sampling points to collect soil in Anshan tailings. After air drying, mixing, drying and sieving, soil samples were digested by microwave method. Concentration of heavy metal in soil samples was measured by inductively coupled plasma-atomic emission spectrometry.14Single factor contaminant index and Nemerow composite index were applied to assess heavy metal pollution.16 The formula is as follows:
| Pi = Ci/Si, PN = {[(Ci/Si)max2 + (Ci/Si)ave2]}1/2 |
2.3. Simulated acid rain and leaching
Based on the characteristics of acid rain in northeastern China,15 we used H2SO4 and HNO3 to formulate acid rain (SO42−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NO3−, 10
NO3−, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). pH value of simulated acid rain is set to 1.0, 3.0, 5.0. Self-made soil column leaching apparatus, soil column cylinder and underpinning device consists of soil column cylinder and underpinning device. Circular cylindrical plexiglass column (height 50 cm, inner diameter 8.0 cm, outer diameter 9.0 cm) was filled with 30 centimeters high air-dried soil. According to climate data of Anshan,16 annual average precipitation is about 760 mm. Annual rainfall is equivalent to 3818 milliliter leaching volume. Leaching experiment was operated for five days to simulate one year's rainfall. The speed was about 65 ml h−1, 12 hours per day.
1). pH value of simulated acid rain is set to 1.0, 3.0, 5.0. Self-made soil column leaching apparatus, soil column cylinder and underpinning device consists of soil column cylinder and underpinning device. Circular cylindrical plexiglass column (height 50 cm, inner diameter 8.0 cm, outer diameter 9.0 cm) was filled with 30 centimeters high air-dried soil. According to climate data of Anshan,16 annual average precipitation is about 760 mm. Annual rainfall is equivalent to 3818 milliliter leaching volume. Leaching experiment was operated for five days to simulate one year's rainfall. The speed was about 65 ml h−1, 12 hours per day.
2.4. Toxicity evaluation
FeSO4 and MnSO4 are used for toxicology experiment. According to the concentration of Fe and Mn dissolved by acid rain, we set up three concentrations to evaluate the effect of Fe and Mn on swimming behavior of Danio rerio in 24 h exposure by using an online monitoring system17 which was built in the Research Center for Eco-Environmental Science, Chinese Academy of Sciences. Three test Danio rerio were placed in a flow-through test chamber, which is closed off on both sides with nylon nets. Eight test chambers were used for each treatment. No food was added during the experiments. One pair of electrodes at the walls of the test chambers send a high frequency signal of alternating current, which is received by a second pair of non-current-carrying electrodes. Behavior strength of Danio rerio was transformed by an A/D transformer and the signal changes formed by the A/D transformer were analyzed automatically by software installed on the equipment (Fig. 1).18 Swimming behavior is sampled automatically during the exposure by OMS every second, and the average behavior strength every 6 min is used to analyze behavioral changes by comparing sample values.After the stress treatment, the whole fish were applied to measuring enzyme activity. SOD and GPX activities were determined in accordance with each kit instructions, finally presented by relative activity (relative activity = sample group OD/control group OD × 100%).
2.5. Removal methods
Self-made activated sludge reactor has significant settlement performance. MLSS concentration is about 4 g L−1, sludge load is 0.2 kg (kg d)−1 and the test temperature is 15 to 20 °C. Operation process of the reactor is composed of four stages: influent, reaction, sediment and effluent. Throughout the test phases, different concentration of Fe and Mn was treated by the reactor and calculated the removal rate.3. Results and discussion
3.1. Determination of heavy metal in soil
Concentration measurement results of eleven kinds of heavy metal (Cu, Zn, Cd, Co, Ni, Fe, Mn, Hg, As, Pb, Cr) were shown in Table 2. Data showed that the concentration of most heavy metal in tailing is in line with soil background value. However, the content of Fe and Mn was much higher than soil background value.19 Therefore, it is necessary to evaluate Fe and Mn. Single factor contaminant index and Nemerow composite index were applied to assess heavy metal pollution.20 Reference standard is shown in Table 1. According to single factor contaminant index, content of Fe caused moderate pollution, content of Mn caused high pollution, and other nine kinds of heavy metal were in a clean state. According to composite pollution index of Anshan tailing (PN = 2.09), it has caused moderate pollution. The main ingredients of pollution were Fe and Mn, which should be thoroughly studied and evaluated.| Single factor contaminant index | Composite pollution index (PN) | Pollution Level |
|---|---|---|
| Pi < 0.7 | PN < 0.7 | Clean |
| 0.7 < Pi < 1 | 0.7 < PN < 1 | Warning value |
| 1 < Pi < 2 | 1 < PN < 2 | Light pollution |
| 2 < Pi < 3 | 2 < PN < 3 | Moderate pollution |
| Pi > 3 | PN > 3 | High pollution |
| Item | Quantity | Background value | Single factor contaminant index | Composite pollution index (PN) |
|---|---|---|---|---|
| Cu, mg kg−1 | 10 | 22.6 | 0.44 | PN = 2.09, moderate pollution |
| Zn, mg kg−1 | 49 | 74.2 | 0.66 | |
| Cd, mg kg−1 | 0.066 | 0.097 | 0.68 | |
| Co, mg kg−1 | <5 | 12.7 | 0.39 | |
| Ni, mg kg−1 | 11 | 26.9 | 0.41 | |
| Fe, % | 6.26 | 2.94 | 2.12 | |
| Mn, mg kg−1 | 2.03 × 103 | 583 | 3.48 | |
| Hg, mg kg−1 | 0.003 | 0.065 | 0.05 | |
| As, mg kg−1 | 5.81 | 11.2 | 0.52 | |
| Pb, mg kg−1 | 7.5 | 26 | 0.29 | |
| Cr, mg kg−1 | 36 | 61 | 0.59 |
3.2. Results of simulated acid rain
Release effects of Fe and Mn in soil samples were tested by the method of simulated acid rain (pH = 1, 3 and 5), showing different dissolution regulation. Most heavy metal release much at low pH, because reduced pH of the system can result in dissolution of carbonate and other combined heavy metal.21 In short, heavy metal in soil will be released through acid rain, leading to heavy metal pollution.Leaching results of Fe and Mn were shown in Table 3. Dissolution of Mn increased with decreasing pH, but dissolution regulation of Fe was relatively complex. Leaching rate of Fe and Mn was shown in Fig. 2. At different pH, leaching rate of Mn was higher than Fe. However, due to high content of Fe in the soil, dissolution of Fe was much more than Mn.
| pH 1.0 | pH 3.0 | pH 5.0 | |
|---|---|---|---|
| Fe concentration (mg L−1) | 99.35 ± 0.71 | 1.87 ± 0.06 | 9.60 ± 0.42 |
| Fe dissolution (mg kg−1) | 993.50 | 18.70 | 96.00 |
| Leaching rate of Fe (‰) | 15.87 | 0.30 | 1.53 |
| Mn concentration (mg L−1) | 16.54 ± 0.53 | 0.60 ± 0.04 | 0.36 ± 0.03 |
| Mn dissolution (mg kg−1) | 165.40 | 6.00 | 3.60 |
| Leaching rate of Mn (‰) | 81.48 | 2.96 | 1.77 |
Leaching rate of Mn was high at pH 1.0, dramatically reduced at pH 3.0, and decreased with increasing pH value. Dissolution of Mn was related to adsorption–desorption and deoxidation. At low pH value, large amount of H+ released to soil, which is beneficial to desorption of manganese ions, aluminum oxides or hydroxides, furthermore, conducive to deoxidation dissolution of Mn.22
Release of Fe is related to precipitation–dissolution.23 At acidic conditions, Fe2+ was released. Leaching rate of Fe was high at pH 1.0, dramatically reduced at pH 3.0, and then slowly increased with the increasing pH value. At pH 1.0, strong acidity intensified weathering reaction of primary minerals. At pH 3.0, release of Fe was based on ion exchange reaction. At pH 5.0, dissolved leaching was the main reaction.
3.3. Joint toxicity evaluation of Fe and Mn
In test of acute toxicity, mortality rate increased with increasing concentration of heavy metal, showing a dose–effect relationship. 24 hours 50% lethal concentration (24-LC50) of Fe2+ is 52 mg L−1. 24 hours 50% lethal concentration (24-LC50) of Mn2+ is 196 mg L−1. According to previous research, it takes 0.1 times of LC50 as a safe concentration.24 Test group settings, 50% lethal concentration and safe concentration were shown in Table 4. In group of T1, T2 and T3, concentration of Mn was in a safe condition.| Concentration (mg L−1) | T1 | T2 | T3 | Control | 24-LC50 | Safe concentration |
|---|---|---|---|---|---|---|
| Fe2+ | 99.35 | 9.60 | 1.87 | 0 | 52 | 5.2 |
| Mn2+ | 16.54 | 0.36 | 0.60 | 0 | 196 | 19.6 |
Behavioral responses of Danio rerio under the exposure to Fe and Mn were shown in Fig. 3. The results showed that Fe and Mn were highly toxic to Danio rerio. Behavior strength of the control group was in a normal state around 0.37, however, the test groups became abnormal. In T1 group, concentration of Fe was much higher than safe concentration, close to 2 times of LC50. Behavior strength was decreasing significantly in short time, because Danio rerio can not adapt to serious pollution. In T2 group, concentration of Fe was higher than safe concentration close to 2 times of safe concentration. Behavior strength showed evident ups-and-downs, abnormal phenomenon emerging such as larger swimming magnitude, accelerated mobile rate and increased respiratory rate caused by a special emergency behavior of Danio rerio called avoidance-behavior. Behavior strength of test group, which was lower than control group, declined after the first rise. Danio rerio adapted to the surroundings through adjustment. In T3 group, behavior strength was similar with control group, because concentration of heavy metal in T3 group was in a safe condition.
Relative activity of Danio rerio exposed to Fe and Mn was shown in Fig. 4. Inhibition rate of SOD and GPX was more than 40% in T1 group, showing evident toxic effect. Relative activity of GPX was lower than SOD, indicating that inhibition effect of GPX was more obvious. Relative activity of GPX decreased with the increasing concentration of heavy metal. Relative activity of SOD showing complicated laws, increased in low concentration, but decreased in high concentration.
Relative activity of SOD and GPX are consistent with behavior adjustment–adaptation of Danio rerio. In low concentration groups, behavior strength increased due to avoidance-behavior. Relative activity of SOD and GPX increased because emergency system is activated to remove free radicals in low concentration. In high concentration groups, behavior strength and relative activity were significantly decreased.
The study showed that Fe and Mn have a strong toxic effect on aquatic organisms. Although Fe and Mn in some groups were lower than safe concentration, but considering excessive heavy metal in tailing with prolonged acid rain, it can cause the accumulation of heavy metal in surface water and ground water, finally deteriorate to serious pollution.
3.4. Removal of Fe and Mn by activated sludge
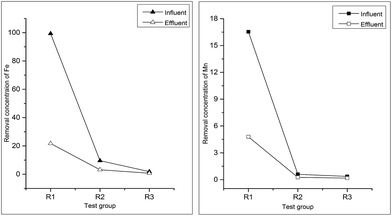
Removal concentration of Fe and Mn by activated sludge was shown in Table 5 and Fig. 5. Removal effect of Fe and Mn was remarkable, furthermore, removal rate was more than 50% at lower concentration. As shown in Fig. 5, the removal rate increased, with the increasing of the concentration. Removal rate of Fe was higher than Mn because of the higher influent concentration of Fe (Fig. 6).| R1 | R2 | R3 | |
|---|---|---|---|
| Influent concentration of Fe (mg L−1) | 99.35 | 9.60 | 1.87 |
| Effluent concentration of Fe (mg L−1) | 21.73 ± 0.57 | 3.17 ± 0.14 | 0.75 ± 0.05 |
| Removal rate of Fe (%) | 78.13 | 66.98 | 59.89 |
| Influent concentration of Mn (mg L−1) | 16.54 | 0.60 | 0.36 |
| Effluent concentration of Mn (mg L−1) | 4.79 ± 0.49 | 0.26 ± 0.04 | 0.17 ± 0.02 |
| Removal rate of Mn (%) | 71.04 | 56.67 | 52.78 |
Removal of Fe and Mn by activated sludge is a complex process. Removal rate was proportional to the initial concentration of heavy metal in water, and related to types of heavy metal. Treatment process, parameters of raw water and microbial metabolic activity also have effect on removal effect.25
Compared with the traditional oxidation method, removal effect of activated sludge is higher, but lower than biological technology.26 Activated sludge is cheap and easily available. We can use activated sludge to remove iron and manganese in water. Activated sludge which is used to treat heavy metal of sewage, can not make heavy metal destroy. Therefore, after treatment of sewage, the remaining sludge should be disposed properly to prevent secondary pollution of heavy metal.27 Graphene oxide has high surface area and functionalized surfaces. The high dispersion property of graphene oxide nanosheets is favorable for the surface oxygen functional groups to freely form strong complexes with metal ions.28 The incorporation of graphene with activated sludge may solve above problems and enhance removal effect.29
4. Conclusion and future work
Content of Fe and Mn in Anshan tailings is much higher than normal, thus causing soil pollution, and seriously affecting the growth of plants. It is also likely to cause bioaccumulation and biomagnification. Acid rain is serious in Liaoning Province, so heavy metal may be activated by acid rain, migrating and transforming from soil into water, resulting in aquatic pollution.Behavior strength and enzyme activities of Danio rerio were applied to study toxicological characteristics of Fe and Mn in water, showing a higher consistency. It proves that Fe and Mn have a strong toxic effect on aquatic organisms, threatening aquatic ecosystem. Behavioral response can be used to establish an on-line monitoring method to monitor water pollution in real time.
Activated sludge was firstly used to treat water polluted by Fe and Mn. It shows that activated sludge can effectively remove heavy metal in sewage, providing a cheap and efficient method to deal with heavy metal contamination of waste water. However, considering that heavy metal could not be degraded by activated sludge, remaining sludge need to be handled properly.
Much work need to be done in the combination of activated sludge and graphene oxide, which will improve the absorption of heavy metals from aqueous systems.30 The absorption mechanism remains to be characterized by kinetic analysis, thermodynamic analysis and spectroscopic techniques.31
Acknowledgements
This study was financially supported by Anshan Soil Project. The authors would like to thank Shandong University and Shandong Normal University for basic research. The authors also wish to thank the anonymous reviewers for providing very helpful comments on the manuscript.References
- M. J. González-Muñoz, M. A. Rodríguez, S. Luque and J. R. Álvarez, Desalination, 2006, 200, 742–744 CrossRef.
- S. Salati and F. Moore, Environ. Monit. Assess., 2010, 164, 677–689 CrossRef CAS PubMed.
- W. U. Bin, S. Y. Zang and N. A. Xiao dong, J. Saf. Environ., 2012, 12, 134–137 Search PubMed.
- J. Dignon and S. Hameed, J. Air Pollut. Control Assoc., 1989, 39, 180–186 CrossRef CAS.
- T. Wood and F. H. Bormann, Water, Air, Soil Pollut., 1977, 7, 479–488 CrossRef CAS.
- X. Zhang, H. Jiang, J. Jin, X. Xu and Q. Zhang, Atmos. Environ., 2012, 46, 590–596 CrossRef CAS.
- Y. Sun, D. Shao, C. Chen, S. Yang and X. Wang, Environ. Sci. Technol., 2013, 47, 9904–9910 CrossRef CAS PubMed.
- M. Menon, S. Hermle, M. S. Günthardt-Goerg and R. Schulin, Plant Soil, 2007, 297, 171–183 CrossRef CAS.
- E. J. Szkokan-Emilson, B. Kielstra, S. Watmough and J. Gunn, Biogeochemistry, 2013, 116, 131–145 CrossRef CAS.
- H. K. Jensen, F. Konradsen and A. Dalsgaard, J. Toxicol., 2011, 2011, 8 Search PubMed.
- T. M. Beasley and H. V. Lorz, J. Environ. Radioact., 1986, 3, 1–22 CrossRef CAS.
- G. Andreas and W. Lutz, J. Plant Nutr. Soil Sci., 2000, 163, 381–385 CrossRef.
- B. G. Oliver and E. G. Cosgrove, Water Res., 1974, 8, 869–874 CrossRef CAS.
- V. L. Dressler, D. Pozebon and A. J. Curtius, Spectrochim. Acta, Part B, 1998, 53, 1527–1539 CrossRef.
- P. Zhou, Y. Zhao, Z. Zhao and T. Chai, J. Environ. Chem. Eng., 2015, 3, 2569–2579 CrossRef CAS.
- W. M. Angevine, J. Geophys. Res.: Atmos., 2015, 104, 30947–30963 CrossRef.
- Z. Ren, N. Su, M. Miao, R. Fu and G. Zhang, J. Biobased Mater. Bioenergy, 2012, 6, 678–681 CrossRef CAS.
- Z. Ren, X. Zhang, X. Wang, P. Qi, B. Zhang, Y. Zeng, R. Fu and M. Miao, Chemosphere, 2015, 120, 252–257 CrossRef CAS PubMed.
- X. Li, L. Liu, Y. Wang, G. Luo, X. Chen, X. Yang, M. H. P. Hall, R. Guo, H. Wang and J. Cui, Geoderma, 2013, 192, 50–58 CrossRef CAS.
- G. C. jun, A. Qiong and D. Y. hua, Rural Eco-Environ., 2005, 21, 66 Search PubMed.
- N. S. Bolan, D. C. Adriano and D. Curtin, Adv. Agron., 2003, 78, 215–272 CAS.
- D. Curtin, J. Ryan and R. A. Chaudhary, Soil Sci. Soc. Am. J., 1980, 44, 947–950 CrossRef CAS.
- A. A. Pohlman and J. G. M. Coll, J. Environ. Qual., 1986, 15, 86–92 CrossRef CAS.
- L. J. Zhou, H. X. Zhan, W. U. Xing bing, H. Yan and Y. F. Zou, Rep. Freshwater Fish. Exp. Stn., Ibaraki Prefect., 2012, 42, 26–30 Search PubMed.
- A. C. Rossin, R. M. Sterritt and J. N. Lester, An International Journal of Environmental Pollution, Water, Air, Soil Pollut., 1982, 17, 185–198 CrossRef CAS.
- D. Ellis, C. Bouchard and G. Lantagne, Desalination, 2000, 130, 255–264 CrossRef CAS.
- F. Pagnanelli, S. Mainelli, L. Bornoroni, D. Dionisi and L. Toro, Chemosphere, 2009, 75, 1028–1034 CrossRef CAS PubMed.
- G. Zhao, J. Li, X. Ren, C. Chen and X. Wang, Environ. Sci. Technol., 2011, 45, 10454–10462 CrossRef CAS PubMed.
- G. Zhao, T. Wen, C. Chen and X. Wang, RSC Adv., 2012, 2, 9286 RSC.
- Y. Sun, S. Yang, Y. Chen, C. Ding, W. Cheng and X. Wang, Environ. Sci. Technol., 2015, 49, 4255–4262 CrossRef CAS PubMed.
- S. Yu, X. Wang, X. Tan and X. Wang, Inorg. Chem. Front., 2015, 2, 593–612 RSC.
| This journal is © The Royal Society of Chemistry 2016 |