Synthesis and properties of azulene-functionalized BODIPYs†
Lizhi
Gai
a,
Jingzhi
Chen
a,
Yue
Zhao
a,
John
Mack
*b,
Hua
Lu
*c and
Zhen
Shen
*a
aState Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Nanjing University, Nanjing, 210093, P. R. China. E-mail: zshen@nju.edu.cn
bDepartment of Chemistry, Rhodes University, Grahamstown 6140, South Africa. E-mail: j.mack@ru.ac.za
cKey Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, 311121, P. R. China. E-mail: hualu@hznu.edu.cn
First published on 22nd March 2016
Abstract
A series of azulene-functionalized BODIPY derivatives have been synthesized via a Suzuki–Miyaura cross-coupling reaction. The introduction of 2-azulenyl moieties onto the BODIPY core results in more red-shifted absorption bands than those observed for 1-azulenyl functionalization. Upon protonation by TFA, a blue shift of the main absorption band of the 2-azulenyl-substituted compounds is observed along with a decrease in intensity, and a new weaker peak is observed at long wavelength. In contrast, the absorption of the 1-azulenyl-substituted compounds is almost unchanged upon protonation.
1. Introduction
Boron-dipyrromethene (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene, BODIPY) dyes have been the focus of intense research interest over the last two decades, due to their highly advantageous photophysical properties, such as their large molar absorption coefficients, narrow absorption and emission bands, and high fluorescence quantum yields that range up to unity.1 BODIPYs have found widespread application as fluorescent probes, biochemical labels, fluorescent switches, light-emitting diodes, photosensitizers for solar cell, photodynamic therapy, and laser dyes.2 Furthermore, their photophysical properties can be fine-tuned through substitution with other functional groups, fused-ring-expansion of the pyrrole moiety and aza-substitution at the meso-carbon position.1Transition metal-catalyzed cross-coupling reactions provide an easy and effective way to achieve 2,6-functionalized BODIPYs and can be used to introduce cyclic π-systems as peripheral substituents.3,4 Azulene (C10H8) can be viewed as a 10-π-electron isomer of naphthalene in which an electron-rich five-membered ring is fused with an electron-deficient seven-membered ring to form resonant tropylium cation and cyclopentadienide anion substructures (Fig. 1).5 The unusual electronic structure results in the formation of a dipolar structure with a permanent dipole moment of around 1.08 D and weak S2 → S0 fluorescence.6–8 Azulene derivatives have been widely used in various areas of molecular materials, such as liquid crystals, conducting polymers, optoelectronic molecular switches, anion receptors, and nonlinear optical (NLO) materials.9–12 We describe here a series of azulene-functionalized BODIPYs prepared via Suzuki–Miyaura cross-coupling reactions to develop new functional molecular-based materials. The impact of introducing 1- and 2-azulenyl units on the electronic structure and pH sensitivity of the dyads has been investigated through a comparison of the optical spectral data and the results of time-dependent DFT (TD-DFT) calculations.
2. Experimental
2.1 Materials and instrumentation
All reagents were obtained from commercial suppliers and used without further purification unless otherwise indicated. All air and moisture-sensitive reactions were carried out under nitrogen atmosphere. Toluene was used after distillation. 1H and 13C NMR spectra were recorded on a Bruker DRX400 spectrometer and referenced to the residual proton signals of the solvent. HR-MS data were recorded on a Bruker Daltonics Apex-III spectrometer. Mass spectra were measured on a Bruker Daltonics AutoflexII™ MALDI–TOF spectrometer.2.2 Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to afford the desired product as a golden solid in 73% yield. 1H NMR (400 Hz, CDCl3): δ 8.27 (d, J = 9 Hz, 2H), 7.54–7.49 (m, 4H), 7.35 (d, J = 6.5 Hz, 2H), 7.28–7.16 (m, 4H), 6.03 (s, 1H), 2.75 (s, 3H), 2.60 (s, 3H), 1.53 (s, 3H), 1.40 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 156.1, 155.1, 143.6, 143.4, 142.0, 140.3, 139.6, 136.5, 135.4, 129.4, 129.2, 128.2, 123.6, 121.7, 118.5, 14.83, 14.62, 14.09, 13.22. UV/Vis (CH2Cl2): λmax (ε) = 528 nm (84
1) as eluent to afford the desired product as a golden solid in 73% yield. 1H NMR (400 Hz, CDCl3): δ 8.27 (d, J = 9 Hz, 2H), 7.54–7.49 (m, 4H), 7.35 (d, J = 6.5 Hz, 2H), 7.28–7.16 (m, 4H), 6.03 (s, 1H), 2.75 (s, 3H), 2.60 (s, 3H), 1.53 (s, 3H), 1.40 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 156.1, 155.1, 143.6, 143.4, 142.0, 140.3, 139.6, 136.5, 135.4, 129.4, 129.2, 128.2, 123.6, 121.7, 118.5, 14.83, 14.62, 14.09, 13.22. UV/Vis (CH2Cl2): λmax (ε) = 528 nm (84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dm3 mol−1 cm−1); HR-MS: calcd for C29H25BF2N2 [M + Na]+: 473.1971; found 473.1976.
000 dm3 mol−1 cm−1); HR-MS: calcd for C29H25BF2N2 [M + Na]+: 473.1971; found 473.1976.
1b was obtained as a golden solid in 68% yield by following a procedure similar to that of 1a. 1H NMR (400 Hz, CDCl3): δ 8.31 (d, J = 9 Hz, 1H), 7.92 (d, J = 9.5 Hz, 1H), 7.73 (d, J = 4 Hz, 1H), 7.59 (t, J = 9.5, 19.5 Hz, 1H), 7.52–7.42 (m, 4H), 7.39–7.35 (m, 2H), 7.19–7.11 (m, 2H), 6.01 (s, 1H), 2.60 (s, 3H), 2.44 (s, 3H), 1.41 (s, 3H), 1.21 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 154.8, 154.6, 142.6, 141.9, 140.7, 139.8, 138.4, 137.2, 136.3, 135.4, 134.2, 130.6, 129.3, 129.2, 128.5, 127.9, 127.8, 123.6, 123.1, 121.4, 120.9, 117.5, 14.3, 14.0, 13.4, 12.8. UV/Vis (CH2Cl2): λmax (ε) = 517 nm (53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dm3 mol−1 cm−1); HR-MS: calcd for C29H25BF2N2 [M + Na]+: 473.1971, found 473.1978.
000 dm3 mol−1 cm−1); HR-MS: calcd for C29H25BF2N2 [M + Na]+: 473.1971, found 473.1978.
2a was obtained as a green solid in 71% yield by following a procedure similar to that of 1a (azulene-Bpin (0.22 mmol), diiodo-BODIPY (0.1 mmol) and Pd (PPh3)4 (0.02 mmol)). 1H NMR (500 Hz, CDCl3): δ 8.29 (d, J = 12 Hz, 4H), 7.58–7.50 (m, 5H), 7.43 (d, J = 2.5 Hz, 2H), 7.41–7.30 (m, 4H), 7.22–7.17 (m, 4H), 2.79 (s, 6H), 1.26 (s, 6H). 13C NMR (100 MHz, CDCl3): δ 157.7, 155.8, 152.6, 146.0, 143.5, 140.5, 139.4, 136.7, 136.0, 129.7, 128.5, 123.8, 118.7, 118.2, 13.6, 13.5. UV/Vis (CH2Cl2): λmax (ε) = 564 nm (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dm3 mol−1 cm−1); MALDI-TOF: calcd for [C29H26BF2N2]+m/z = 576.49, found m/z = 576.47 [M]+, 557.28 [M − F]+.
000 dm3 mol−1 cm−1); MALDI-TOF: calcd for [C29H26BF2N2]+m/z = 576.49, found m/z = 576.47 [M]+, 557.28 [M − F]+.
2b was obtained as a green solid in 63% yield by following a procedure similar to that of 2a. 1H NMR (500 Hz, CDCl3): δ 8.34 (d, J = 9.5 Hz, 2H), 7.97 (dd, J = 6.9, 6.7 Hz, 2H), 7.78 (d, J = 3.3 Hz, 2H), 7.60 (t, J = 9.8, 19.6 Hz, 2H), 7.52–7.45 (m, 2H), 7.46–7.44 (m, 4H), 7.21–7.13 (m, 5H), 2.48 (s, 6H), 1.54 (s, 6H). 13C NMR (100 MHz, DMSO-d6): δ 154.5, 141.9, 139.7, 138.5, 138.4, 137.2, 136.3, 135.4, 135.3, 134.5, 130.9, 129.4, 129.3, 128.5, 128.0, 123.7, 123.1, 121.0, 117.5, 30.9, 22.0, 13.9, 13.5, 13.0, 12.9. UV/Vis (CH2Cl2): λmax (ε) = 551 nm (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dm3 mol−1 cm−1); HR-MS: calcd for C39H31BF2N2 [M + Na]+: 599.2441; found 599.2447.
000 dm3 mol−1 cm−1); HR-MS: calcd for C39H31BF2N2 [M + Na]+: 599.2441; found 599.2447.
2.3 X-ray structure determination
The X-ray crystallography data for 1a were collected on a Rigaku Saturn CCD diffractometer by using graphite monochromated Mo Kα radiation (λ = 0.71073 Å) in the ω–2θ scan mode. The structure was solved by direct methods and refined on F2 by using the full-matrix least-squares methods of the SHELX program.14 The X-ray structure calculations and the generation of the associated molecular graphics were carried out using the SHELX-97 and Diamond programs, respectively. 1a: C29H25BF2N2; a red block-like crystal of approximate 0.28 × 0.26 × 0.22 mm3 dimensions was measured. Space group P21/n, a = 16.204(3) Å, b = 19.565(4) Å, c = 7.4534(17) Å, α = 90°, β = 90°, γ = 90°, V = 2363.0(9) Å3, Z = 4, F(000) = 944, ρ = 1.26575 g cm−3, R1 = 0.0859, wR2 = 0.1120, GOF = 0.980. CCDC no. 1415532 contains the supplementary crystallographic data for this paper.2.4 Spectroscopic measurements
UV-visible absorption spectra were recorded on a Shimadzu UV-2550 spectrophotometer. Fluorescence spectra were measured on a Hitachi F-4600 FL spectrophotometer with a xenon arc lamp as light source. Samples for absorption and emission measurements were contained in 1 × 1 cm quartz cuvettes. For all measurement, the temperature was kept constant at (298 ± 2) K.2.5 Computational details
The ground state structures of dyads and the corresponding protonated species were optimized using the density functional theory (DFT) method with the B3LYP functional and 6-31G(d) basis sets. The absorption properties were predicted by time-dependent (TD-DFT) method by using the CAM-B3LYP functional with the same basis sets. All of the calculations were performed with the Gaussian09 program package.133. Results and discussion
3.1 Synthesis
The synthetic strategy for the azulenyl-substituted BODIPYs is shown in Scheme 1. 2-Iodo-and 2,6-diiodo-substituted 1,3,5,7-tetramethyl-BODIPYs (4a and 4b) were prepared according to the reported procedures.3c1a and 2a were synthesized from 2-iodo substituted 4a in toluene, while 1b and 2b were synthesized from 2,6-diiodo substituted BODIPY 4b, through a Suzuki–Miyaura cross-coupling reaction with 2-boryl 3a (or 1-boryl 3b) azulene by using Pd(PPh3)4 as a catalyst.15 The dyes were formed in 73, 68, 71 and 63% yield, respectively (Scheme 1). All of the new compounds were characterized by 1H and 13C NMR spectroscopy and HR-MS. The dyes are highly soluble in commonly-used organic solvents at room temperature.3.2 Crystal structure
A single crystal of 1a was obtained by slow diffusion of hexane into a CH2Cl2 solution. The crystal structure of 1a is shown in Fig. 2. The boron atom is coordinated in a tetrahedral geometry by two nitrogen and two fluorine atoms (ESI,† Table S2). The indacene moiety is highly planar with an average root-mean-square (rms) deviation of 0.027 Å. The torsion angles between the indacene plane on the one hand and the meso-phenyl and azulene rings on the other, are 83.6 and 37.5°, respectively, due in each case to the steric interactions with the adjacent methyl substituents. 1a is centrosymmetric, and the shortest π–π distance between neighboring BODIPY core structures is 4.38 Å. A well-ordered molecular packing is a critical factor in developing electron transporting materials. No attempt was made to estimate the transfer integral following the approach of Valeev and coworkers,16 since the HOMO of the BODIPY core mixes extensively with close-lying MOs that are localized primarily on the azulene substituents, so it is not safe to assume that the HOMO and HOMO−1 of a dimer formed using the crystal structure are derived exclusively from the HOMO of the monomer. | ||
| Fig. 2 ORTEP drawings of the molecular structures of 1a with thermal ellipsoids set at 50% probability (a) top view; (b) side view; (c and d) crystal packing diagram of 1a. | ||
3.3 Spectroscopic properties in solution
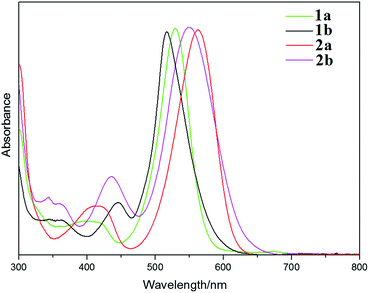
The absorption spectra of 1a, 1b, 2a and 2b were measured in CH2Cl2 (Fig. 3). The azulenyl-BODIPYs have their main band maxima in the 500–600 nm region, which can be assigned as intense 0–0 maxima of the S0 → S1 transition. Weaker and broader bands in the 350–450 nm region have been attributed to the S0 → S2 transition of the BODIPY chromophore.17 The main absorption bands of di-substituted dyes 2a and 2b are red-shifted by around 30 nm relative to the corresponding bands of mono-substituted dyes 1a and 1b. In each case, there is a significant increase in the full width at half-maximum (fwhmabs).The UV-visible absorption spectra of 1a, 1b, 2a and 2b were also measured in hexane, dichloromethane, toluene and THF (ESI,† Fig. S1). The absorption maxima of the compounds are almost independent of solvent polarity with only a small variation of 2–3 nm observed upon going from THF to hexane, which is consistent with what is normally observed for BODIPY dyes.1a It is noteworthy that the main spectral bands of the 2-azulenyl substituted dyes, 1a and 2a, are more red-shifted than those of the corresponding 1-azulenyl substituted dyes, 1b and 2b. In contrast, a blue-shift is predicted in the shorter wavelength absorption maxima of 1b and 2b in the 380–470 nm region.
In order to gain further insight into the influence of the azulenyl group on the properties of the BODIPY derivatives, the effect of protonation was examined (Fig. 4). Upon addition of TFA, the main absorption of 1a and 2a are blue-shifted and there is a decrease in the molar extinction coefficient of the band maximum due to generation of azulenium cations.18 The second higher energy maximum that is characteristic of the azulene rings, shifts from 421 to 372 nm due to the large dihedral angle between the BODIPY core and the azulenium cation. A new peak is observed at 612 nm for 1a and 640 nm for 2a. In contrast, there is no obvious change for the absorption spectra of 1b and 2b upon the addition of excess TFA. Upon addition of trimethylamine, the absorption spectrum of 1b is not regenerated, presumably due to the decomposition of an unstable protonated azulene species.19 Since azulenes have relatively larger HOMO–LUMO gaps, they are generally not suitable for use as molecular probes for the detection of ions by the naked eye. However, when azulenes are substituted onto the BODIPY core marked spectral changes in the visible region are observed when 1a and 2a react with TFA (Fig. 4), making these compounds potentially suitable for use as pH sensors.
 | ||
| Fig. 4 Changes in the UV-visible absorption spectra of azulenyl-BODIPY derivatives 1a, 1b, 2a, 2b in dichloromethane upon the addition of TFA. | ||
The dyes exhibit extremely weak fluorescence. This may be due to the formation of a charge separated state due to intramolecular charge transfer, since this would result in an enhancement in the rate of non-radiative decay.
3.4 Theoretical calculations13
To gain a deeper understanding of the spectroscopic and electronic properties of 1a, 1b, 2a and 2b and the protonated cationic species of 1a and 2a, geometry optimizations were performed using density functional theory (DFT) by using the B3LYP functional of the Gaussian 09 program with 6-31G(d) basis sets. TD-DFT calculations were carried out with the CAM-B3LYP functional and 6-31G(d) basis sets. In the B3LYP optimized structure of 1a, the dihedral angle between the azulene ring and the BODIPY core is 41.6°, which correlates well with that of the single crystal data (37.5°). The dihedral angles calculated for the azulene ring and BODIPY core structure of the 2-position substituted dyes 1a and 2a have smaller angles than the 1-position substituted dyes 1b and 2b, since there is less scope for steric interactions with the methyl groups at the 2, 3, 5 and 7-positions of the BODIPY core (Table 1 and ESI,† Table S1).| 1a | 1b | 2a | 2b | 1a + H+ | 2a + H+ | |
|---|---|---|---|---|---|---|
| θ | 41.6° | 59.8° | 41.4° | 60.3° | 9.9° | 9.3°, 20.6° |
For 1a, 1b, 2a and 2b, the HOMO orbital is located over the whole azulene-BODIPY core structure (Fig. 5), whereas the corresponding LUMOs are localized on the BODIPY core structure. It is obvious on this basis that there is significant charge transfer from the azulenyl groups to the central fluorophore involved in forming the S1 excited state. This could lead to the observed quenching of the fluorescence via an intramolecular charge transfer (ICT) mechanism, since a charge-separated state can be formed (Fig. 5, ESI Table S1†). The TD-DFT calculations (Fig. 6) predict the presence of low-lying forbidden charge transfer bands, which provides another possible explanation for the quenching. The HOMO and LUMO of 1b and 2b are destabilized relative to those of 1a and 2a due to an increase in the electron donating inductive effect on moving from 2-azulenyl to 1-azulenyl substituents (Fig. 5). Although there is a slight narrowing of the predicted HOMO–LUMO gaps due to a relative destabilization of the HOMOs of 1b and 2b (Fig. 5), there is a blue shift of the main absorption bands of 1b and 2b in both the experimental and calculated spectra (Fig. 5 and 6). Upon protonation to form (1a and 2a)-H+, the LUMO is largely located on the protonated azulene ring, while the same part of the HOMO has less electron density compared with the neutral state. Although the relative band intensities in the calculated spectra are clearly not accurately reproduced, a blue shift is predicted in the wavelength of the main spectral band of (1a and 2a)-H+ similar to the apparent trend in the experimental data (Fig. 6).
 | ||
| Fig. 6 TD-DFT spectra of 1a, 1b, 2a and 2b (left) and 1a, 2a and their monoprotonated structures (right) calculated with the CAM-B3LYP functional and 6-31G(d) basis sets. Red diamonds are used to denote the bands that are dominated by the HOMO → LUMO transition of the BODIPY chromophore, while yellow and green diamonds are used to highlight bands with charge transfer between the BODIPY and azulene moieties and other BODIPY π → π* bands. The experimental spectra are plotted against a secondary axis. The details of the calculations are provided in ESI Table S2.† | ||
4. Conclusions
In summary, the synthesis, characterization, and theoretical analysis of a series of azulenyl-substituted BODIPY dyes has been investigated. Substitution at the 2-position of the azulene ring results in a larger red-shift of the main spectral band than substitution at the 1-position, and no fluorescence is observed either before or after protonation by TFA. Molecular modeling suggests that the most likely explanation is that there is a dark state with charge transfer character below the lowest energy π–π* state of the BODIPY chromophore.Acknowledgements
Financial support was provided by the Major State Basic Research Development Program of China (Grant no. 2013CB922101 & 2011CB808704), the National Natural Science Foundation of China (NSFC) (no. 21371090) to Z. S. and (21471042) to H. L., the Natural Science Foundation of Jiangsu Province (BK20130054) to Z. S., the Zhejiang Provincial Natural Science Foundation of China (Grant No. LY14B010003) and a NSFC and National Research Foundation of South Africa China-South Africa joint research program (CS08-L07 and UID: 95421) to Z. S. and J. M. Theoretical calculations were carried out at the Centre for High-Performance Computing in Cape Town.Notes and references
- (a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed; (b) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., 2008, 120, 1202 ( Angew. Chem., Int. Ed. , 2008 , 47 , 1184 ) CrossRef; (c) H. Lu, J. Mack, Y. Yang and Z. Shen, Chem. Soc. Rev., 2014, 43, 4778 RSC; (d) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77 RSC; (e) S. Xiao, Q. Cao and F. Dan, Curr. Org. Chem., 2012, 16, 2970 CrossRef CAS; (f) S. G. Awuah and Y. You, RSC Adv., 2012, 2, 11169 RSC; (g) A. B. Nepomnyashchii and A. J. Bard, Acc. Chem. Res., 2012, 45, 1844 CrossRef CAS PubMed.
- (a) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC; (b) L. Bonardi, H. Kanaan, F. Camerel, P. Jolinat, P. Retailleau and R. Ziessel, Adv. Funct. Mater., 2008, 18, 401 CrossRef CAS; (c) A. C. Benniston and G. Copley, Phys. Chem. Chem. Phys., 2009, 11, 4124 RSC; (d) R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496 RSC; (e) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77 RSC; (f) T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162 CrossRef CAS PubMed; (g) A. C. Benniston and G. Copley, Phys. Chem. Chem. Phys., 2009, 11, 4124 RSC; (h) M. Benstead, G. H. Mehl and R. W. Boyle, Tetrahedron, 2011, 67, 3573 CrossRef CAS; (i) J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323 RSC; (j) A. Bessette and G. S. Hanan, Chem. Soc. Rev., 2014, 43, 3342 RSC; (k) B. Verbelen, S. Boodts, J. Hofkens, N. Boens and W. Dehaen, Angew. Chem., Int. Ed., 2015, 54, 4612 CrossRef CAS PubMed; (l) F. Wang, Y. Zhu, L. Zhou, L. Pan, Z. Cui, Q. Fei, S. Luo, D. Pan, Q. Huang, R. Wang, C. Zhao, H. Tian and C. Fan, Angew. Chem., Int. Ed., 2015, 54, 7349 CrossRef CAS PubMed; (m) P. P. Goswami, A. Syed, C. L. Beck, T. R. Albright, K. M. Mahoney, R. Unash, E. A. Smith and A. H. Winter, J. Am. Chem. Soc., 2015, 137, 3783 CrossRef CAS PubMed; (n) C. Zhao, X. Zhang, K. Li, S. Zhu, Z. Guo, L. Zhang, F. Wang, Q. Fei, S. Luo, P. Shi, H. Tian and W. H. Zhu, J. Am. Chem. Soc., 2015, 137, 8490 CrossRef CAS PubMed; (o) E. Palao, T. Slanina, L. Muchová, T. Šolomek, L. Vítek and P. Klán, J. Am. Chem. Soc., 2016, 138, 126 CrossRef CAS PubMed.
- (a) L. Jiao, W. Pang, J. Zhou, Y. Wei, X. Mu, G. Bai and E. Hao, J. Org. Chem., 2011, 76, 9988 CrossRef CAS PubMed; (b) V. Leen, E. Braeken, K. Luckermans, C. Jackers, M. Van der Auweraer, N. Boens and W. Dehaen, Chem. Commun., 2009, 4515 RSC; (c) L. Z. Gai, J. Mack, H. Lu, H. Yamada, D. Kuzuhara, G. Lai, Z. Li and Z. Shen, Chem.–Eur. J., 2014, 20, 1091 CrossRef CAS PubMed; (d) T. Rohand, W. Qin, N. Boens and W. Dehaen, Eur. J. Org. Chem., 2006, 4658 CrossRef CAS.
- (a) A. Poirel, A. De Nicola, P. Retailleau and R. Ziessel, J. Org. Chem., 2012, 77, 7512–7525 CrossRef CAS PubMed; (b) S. Yin, V. Leen, S. V. Snick, N. Boensa and W. Dehaen, Chem. Commun., 2010, 46, 6329 RSC; (c) V. Lakshmi and M. Ravikanth, Dyes Pigm., 2013, 96, 665 CrossRef CAS; (d) L. Jiao, C. Yu, T. Uppal, M. Liu, Y. Li, Y. Zhou, E. Hao, X. Hu and M. G. H. Vicente, Org. Biomol. Chem., 2010, 8, 2517 RSC; (e) V. Leen, T. Leemans, N. Boens and W. Dehaen, Eur. J. Org. Chem., 2011, 4386 CrossRef CAS.
- (a) K. H. Grellmann, E. Heilbronner, P. Seiler and A. Weller, J. Am. Chem. Soc., 1968, 4238 CrossRef CAS; (b) J. Michl and E. W. Thulstrup, Tetrahedron, 1976, 32, 205 CrossRef CAS; (c) A. G. Anderson and B. M. Steckler, J. Am. Chem. Soc., 1959, 81, 4941 CrossRef CAS.
- (a) K.-P. Zeller, Azulene, in Houben-Weyl: Methoden der OrganischenChemie, 4th edn, Georg Thieme, Stuttgart, Germany, 1985, vol. V, part 2C, p. 127 Search PubMed; (b) A. L. Crombie, J. L. Kane, K. M. Shea and R. L. Danheiser, J. Org. Chem., 2004, 69, 8652 CrossRef CAS PubMed.
- (a) P. Foggi, F. V. R. Neuwahl, L. Moroni and P. R. Salvi, J. Phys. Chem. A, 2003, 107, 1689 CrossRef CAS; (b) S. V. Shevyakov, H. Li, R. Muthyala, A. E. Asato, J. C. Croney, D. M. Jameson and R. S. H. Liu, J. Phys. Chem. A, 2003, 107, 3295 CrossRef CAS.
- (a) B. D. Wagner, D. Tittelbach-Helmrich and R. P. Steer, J. Phys. Chem., 1992, 96, 7904 CrossRef CAS; (b) E. A. Dragu, S. Nica, M. Raicopol, A. Baran, D. F. Anghel, B. Cojocaru, L. Tarko and A. C. Razus, Tetrahedron Lett., 2012, 53, 2611 CrossRef CAS; (c) E. Amir, R. J. Amir, L. M. Campos and C. J. Hawker, J. Am. Chem. Soc., 2011, 133, 100486 CrossRef PubMed; (d) S. Ito and N. Morita, Eur. J. Org. Chem., 2009, 27, 4567 CrossRef.
- (a) S. Schmitt, M. Baumgarten, J. Simon and K. Hafner, Angew. Chem., Int. Ed., 1998, 37, 1077 CrossRef; (b) T. Mrozek, H. Gorner and J. Daub, Chem.–Eur. J., 2001, 7, 1028 CrossRef CAS PubMed; (c) J. E. Frey, A. M. Andrews, S. D. Combs, S. P. Edens, J. J. Puckett, R. E. Seagle and L. A. Torreano, J. Org. Chem., 1992, 57, 6460 CrossRef CAS; (d) T. Tang, T. Lin, F. Wang and C. He, Polym. Chem., 2014, 5, 2980 RSC; (e) M. Koch, O. Blacque and K. Venkatesan, J. Mater. Chem. C, 2013, 1, 7400 RSC.
- (a) J. Xia, B. Capozzi, S. Wei, M. Strange, A. Batra, J. R. Moreno, R. J. Amir, E. Amir, G. C. Solomon, L. Venkataraman and L. M. Campos, Nano Lett., 2014, 14, 2941 CrossRef CAS PubMed; (b) F. Wang, Y.-H. Lai and M.-Y. Han, Org. Lett., 2003, 5, 4791 CrossRef CAS PubMed.
- (a) F. Wang, Y.-H. Lai and M.-Y. Han, Macromolecules, 2004, 37, 3222 CrossRef CAS; (b) Y. Yamaguchi, Y. Maruya, H. Katagiri, K. Nakayama and Y. Ohba, Org. Lett., 2012, 14, 2316 CrossRef CAS PubMed; (c) X. Wang, J.-K. Ng, P. Jia, T. Lin, C. M. Cho, J. Xu, X. Lu and C. He, Macromolecules, 2009, 42, 5534 CrossRef CAS.
- (a) F. Wang and Y.-H. Lai, Macromolecules, 2003, 36, 536 CrossRef CAS; (b) S. E. Estdale, R. Brettle, D. A. Dummur and C. M. Marson, J. Mater. Chem., 1997, 7, 391 RSC; (c) Z. Hussain, H. Hopf, L. Pohl, T. Oeser, A. K. Fischer and P. G. Jones, Eur. J. Org. Chem., 2006, 5555 CrossRef CAS.
- M. J. Frisch, et al., Gaussian 09 Search PubMed, see ESI† for full reference and a detail description of the computational methods.
- (a) SHELXTL, version 5.1, Siemens Industrial Automation, Inc., Madison, WI, 1997 Search PubMed; (b) G. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- (a) K. Kurotobi, M. Miyauchi, K. Takakura, T. Murafuji and Y. Sugihara, Eur. J. Org. Chem., 2003, 3663 CrossRef CAS; (b) M. Murai, S.-Y. Ku, N. D. Treat, M. J. Robb, M. L. Chabinyc and C. J. Hawker, Chem. Sci., 2014, 5, 3753 RSC.
- E. F. Valeev, V. Coropceanu, D. A. da Silva Filho, S. Salman and J.-L. Brédas, J. Am. Chem. Soc., 2006, 128, 9882 CrossRef CAS PubMed.
- (a) M. Kollmannsberger, K. Rurack, U. Resch-Genger and J. Daub, J. Phys. Chem. A, 1998, 102, 10211 CrossRef CAS; (b) W. Qin, M. Baruah, M. V. Auweraer, F. C. De Schryver and N. Boens, J. Phys. Chem. A, 2005, 109, 7371 CrossRef CAS PubMed.
- (a) M. Oda, A. Fukuta, T. Kajioka, T. Uchiyama, H. Kainuma, R. Miyatake and S. Kuroda, Tetrahedron, 2000, 56, 9917 CrossRef CAS; (b) P. A. Plattner, E. Heilbronner and S. Weber, Helv. Chim. Acta, 1952, 35, 1036 CrossRef CAS; (c) E. Amir, M. Murai, R. J. Amir, J. S. Cowart Jr, M. L. Chabinyc and C. J. Hawker, Chem. Sci., 2014, 5, 4483 RSC.
- F. Wang, Y.-H. Lai, N. M. Kocherginsky and Y. Y. Koteski, Org. Lett., 2003, 5, 995 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: MS data and 1H NMR spectra, and additional experimental and calculated optical spectra. CCDC 1415532. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra00743k |
| This journal is © The Royal Society of Chemistry 2016 |