Synthesis and properties of novel chiral imidazolium-based ionic liquids derived from carvone†
Abstract
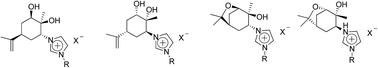
A large series of novel chiral imidazolium ionic liquids were synthesized using the terpenoid carvone as the chiral substrate. Their specific rotations were characterized and their potential use in chiral recognition was demonstrated by studying interactions with racemic Mosher's acid salt.

 Please wait while we load your content...
Please wait while we load your content...