Interaction of cationic gemini surfactant tetramethylene-1,4-bis(dimethyltetradecylammonium bromide) with anionic polyelectrolyte sodium carboxymethyl cellulose, with two different molar masses, in aqueous and aquo-organic (isopropanol) media†
Sibani Das,
Satyajit Mondal and
Soumen Ghosh*
Centre for Surface Science, Department of Chemistry, Jadavpur University, Kolkata – 700032, India. E-mail: gsoumen70@hotmail.com; Fax: +91 33 24146266
First published on 16th March 2016
Abstract
A comparative study of the interactions of the cationic gemini surfactant tetramethylene-1,4-bis(dimethyltetradecylammonium bromide) (14-4-14) with anionic polymer sodium carboxymethylcellulose (NaCMC), with two different molar masses, was performed in water and isopropanol (IP)–water media. The interaction process was studied in detail using conductometry, tensiometry, turbidimetry and viscometry. At very low concentration, 14-4-14 monomers interact with the polymer. Above the critical aggregation concentration (cac), small micelle-like aggregates form complexes with the polymer. During the interaction process, coacervates are formed beyond Cs (polymer saturation concentration), which initially grow by aggregation and stay in the solution throughout the process. The interaction process is affected by addition of isopropanol into the medium. The intrinsic viscosities of the two NaCMCs were determined by using Huggins and Kraemer equations. Dynamic light scattering (DLS) study helps to determine the hydrodynamic size of the dispersed polymer and its surfactant-interacting complexes. The hydrodynamic size of the dispersed polymer and its interacting complex in IP–water media is lower than that obtained in aqueous solution. The surface morphology of the solvent removed polymer and its surfactant-interacting complex were examined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) techniques. The pattern of the morphologies depends on the polymer–surfactant composition and the solvent environment.
1. Introduction
The amphiphilic nature of surfactants makes them essential ingredients of age old technological processes, such as soap, cosmetics, and paper making, etc. Today they are also widely used in gene transfection, preparation of mesoporous materials, phase transfer catalysts, etc.1–5 In addition to this, complex formation between ionic surfactant and oppositely charged polyelectrolyte is a fascinating area of research.6–13 The ternary system of polymer–surfactant–water has drawn considerable attention due to the wide range of domestic and industrial applications such as, detergency, food formulations, paints, drug delivery systems, coatings, cosmetics, enhanced oil recovery, water purification etc.14–23 Polymers are generally used as viscosity modifier and binder. The mixture of polyelectrolytes and surfactants with opposite charges often shows strong tendency to form insoluble complexes and leads to phase separation which may be either liquid–liquid phase separation (coacervation) or solid–liquid phase separation (precipitation).6,9,23 In coacervation process, the colloidal or polymer solution separates into two immiscible liquid phases. This process can be divided into two distinct classes, simple and complex coacervation. Addition of additives (salt, alcohols etc.) to single component colloid solution produced simple coacervation while addition of oppositely charged macromolecular component produced complex coacervation. The important factors for coacervation are polyelectrolyte composition, charge density, chain length, chain flexibility, polymer molecular weight, micelle surface charge density, micelle size, shape, pH and ionic strength of the medium, and so on.6,9,24–26 Coacervation is mainly dominated by suitable balance among electrostatic, hydrophobic and solvent interaction.27,28 The field is enriched everyday by using different combinations of additives, like alcohols (especially, in pharmaceutics and cosmetics) and salts etc. to modify and control the solubility, durability and dispersity of the system. Alcohols are frequently used as cosolvents as well as cosurfactants. As a cosurfactant, alcohol increases the cmc, but, as a cosolvent they increase the hydrophobicity of the medium and hence cmc increases. Isopropanol is used as additives in pharmaceutical and medicinal preparation as it is nontoxic, biofriendly and fairly soluble in water.29,30 All the above factors mutually control the interaction between polyelectrolyte and ionic surfactant. Here is the importance of focusing on the effect of cosolvent (IP) and polymer molar mass in the interaction process.In this work, we have used sodium carboxymethyl cellulose (NaCMC), one of the ionic derivatives of cellulose.31 It is a water soluble linear polymer having β-1,4-linkage between the glycane unit. The structure of NaCMC was shown in Scheme 1 of ESI.†10 It acts as a polyanion at pH > 4. NaCMC is a useful component of many food systems and is widely used as a thickener and binding agent in pharmaceutical applications. Interaction of NaCMC with alkyl ammonium bromide was widely studied;10,32–34 but the interaction of NaCMC with cationic gemini surfactant is rare. Recently, Kabir-ud-Din et al.35 have reported the interaction of cationic gemini surfactants with NaCMC, but their observations were quite different from our study. Actually, gemini surfactants are a novel class of compounds. This class of surfactants impart better surface properties as compared to the conventional single chain surfactants,36–39 such as remarkably low critical micelle concentration (cmc), high surface activity, better wetting ability, unusual aggregation morphologies, low Kraft temperature, unusual rheological properties etc. Having such characteristics, gemini surfactant is superior than conventional single chain surfactant. The interaction between NaCMC and gemini surfactant in aquo-IP medium may be helpful from application point of view. There have been reports on interaction between gemini surfactant and polyelectrolyte, but report on the effect of polymer molar mass and polar organic solvent (isopropanol, IP) on the interaction is rare.
In this work, we have attempted a detailed study on the interaction of cationic gemini surfactant 14-4-14 with anionic polyelectrolyte NaCMC having two different molar masses in aqueous and IP–water medium by tensiometric, conductometric, fluorimetric, turbidimetric and viscometric methods. The hydrodynamic size of the surfactant alone and also the polymer interacted complex were determined by dynamic light scattering (DLS) method. The morphology of the polymer and surfactant–polymer interacted complex were investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) techniques. We also try to focus on the effect of molar mass of the polymer on the interaction process.
2. Experimental section
2.1. Materials
Sodium carboxymethylcellulose (NaCMC) was used AR grade product of Aldrich, with a viscosity-average molecular weight 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (NaCMC-1) and 208
000 (NaCMC-1) and 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (NaCMC-2) and average degree of substitution (average number of carboxymethyl groups per cellulose unit), S = 0.9 and S > 0.4 respectively. The structure of NaCMC was given in the ESI.†
000 (NaCMC-2) and average degree of substitution (average number of carboxymethyl groups per cellulose unit), S = 0.9 and S > 0.4 respectively. The structure of NaCMC was given in the ESI.†
The gemini surfactant tetramethylene-1,4-bis(dimethyltetradecylammonium bromide) (14-4-14) (GS) was synthesized in our laboratory.40 The structure was confirmed by 1H NMR spectroscopy. The purity of the GS was further confirmed by the absence of minima in surface tension (γ) vs. log[surfactant] plot. The structure of the gemini surfactant was depicted in Scheme 1. 1,4-Dibromo butane and N,N-dimethyltetradecyl amine were purchased from Aldrich and used without any further purification. AR grade ethanol (purity = 99.9%) was purchased from Changshu Yangyuan Chemical. Isopropanol (IP) used was an AR grade product of SRL, India. Double distilled water of specific conductance of 2–4 μS cm−1 was used for the preparation of all sample solutions at 298 K.
2.2. Methods
3. Results and discussions
3.1. Interaction of 14-4-14 with sodium carboxymethylcellulose (NaCMC) in aqueous and aqueous-IP medium
Table 1 showed that the cac values were slightly affected by the concentration of polymer. The cac for the interaction of 14-4-14/NaCMC-1 is lower than that of 14-4-14/NaCMC-2 (Fig. 1(b)). In the post-cac region, the aggregates continue to bind with the anionic polymer sites and do not allow the transfer of the cation to the interface. As a result, the constant γ value is maintained. In this stage, the assembly of the surfactant is attached to the backbone of the polymer molecule; only the configuration of the polymer becomes changed. Such type of binding is continued up to Cs, called the polymer saturation concentration. At this higher conc, the micellar aggregates interact strongly with the polymer and the complex goes to the bulk of the solution. After that, the further addition of 14-4-14 helped the amphiphilic molecules to get adsorbed at the interface causing a reduction in γ, which became complete (i.e. saturation of the interface) at Cf (extended cmc). Beyond this point, free micelles were formed in solution and further addition of surfactant molecule does not affect the γ value. In literature, such types of phenomena of polymer–surfactant systems are available.10,32–34,47 The values of Cs and Cf increase with increasing concentration of polymer (Table 1). Again, with increasing the molar mass and hence the degree of substitution (S), the cac value decreases and distance between cac and Cs increases. That was shown in Fig. 1(b). The results were given in Tables 1 and S1.†
| % of IP | [NaCMC-1]/g% | Conductometry | Surface tension | α1 | α2 | −ΔG0m kJ mol−1 | |||
|---|---|---|---|---|---|---|---|---|---|
| Cs (mM) | Cf (mM) | cac (mM) | Cs (mM) | Cf (mM) | |||||
| 0 | 0.005 | 0.060 | 0.264 | 0.005 | 0.058 | 0.270 | 1.33 | 0.56 | 57.1 |
| 0.0075 | 0.073 | 0.306 | 0.006 | 0.069 | 0.280 | 1.24 | 0.63 | 52.2 | |
| 0.01 | 0.082 | 0.325 | 0.012 | 0.092 | 0.337 | 1.11 | 0.67 | 49.6 | |
| 5 | 0.005 | 0.061 | 0.301 | 0.007 | 0.054 | 0.286 | 1.23 | 0.60 | 53.9 |
| 0.0075 | 0.077 | 0.314 | 0.007 | 0.075 | 0.302 | 1.21 | 0.63 | 51.9 | |
| 0.01 | 0.097 | 0.330 | 0.010 | 0.100 | 0.360 | 1.20 | 0.67 | 49.3 | |
| 7 | 0.005 | 0.067 | 0.310 | 0.010 | 0.055 | 0.297 | 1.24 | 0.62 | 52.5 |
| 0.0075 | 0.083 | 0.318 | 0.020 | 0.086 | 0.324 | 1.23 | 0.64 | 51.2 | |
| 0.01 | 0.118 | 0.459 | 0.030 | 0.125 | 0.471 | 1.13 | 0.70 | 46.2 | |
| 10 | 0.005 | 0.070 | 0.340 | 0.013 | 0.063 | 0.314 | 1.20 | 0.63 | 51.4 |
| 0.0075 | 0.084 | 0.373 | 0.021 | 0.085 | 0.357 | 1.16 | 0.66 | 49.3 | |
| 0.01 | 0.128 | 0.483 | 0.036 | 0.129 | 0.482 | 1.12 | 0.71 | 45.3 | |
Now interaction of GS with NaCMC-1 is shown in presence of IP in Fig. 1(c) and (d). Fig. 1(c) shows the higher surface activity of the system of 0.005% NaCMC-1 with 14-4-14 in presence of higher concentration of IP resulting the lower surface tension of the solution. The values of cac, Cs and Cf are dependent on solvent composition and increase with increasing composition of IP content in water. The Cs and Cf were more influenced by IP compared with cac. The interaction between cationic gemini surfactant and anionic polymer form a complex which can self-assemble to form a turbid coacervate phase.6 Coacervates appear after Cs and exist in the solution. These are not dissolved even at higher concentration of surfactant. The rate of formation of coacervation decreases with increasing concentration of IP. The tendency towards phase separation and coacervation region increases with increasing molar mass and surface charge density of the polyelectrolyte. Interfacial parameters, e.g. Gibbs surface excess  , the area of per monomer of 14-4-14
, the area of per monomer of 14-4-14 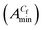 and the surface tension value of the solution at saturated air solution interface, for the free micelle formation of GS between Cs and Cf in the polymer solution are calculated from the following relations:10,42,47
and the surface tension value of the solution at saturated air solution interface, for the free micelle formation of GS between Cs and Cf in the polymer solution are calculated from the following relations:10,42,47
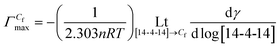 | (1) |
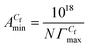 | (2) |
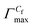 ,
,  and γCf for both systems are presented in Tables 2 and S2.†
and γCf for both systems are presented in Tables 2 and S2.†
 of 14-4-14 with NaCMC-1 in aqueous and aqueous-IP medium at 298 K
of 14-4-14 with NaCMC-1 in aqueous and aqueous-IP medium at 298 K
| % of IP | [NaCMC-1]/g% | 106 mol m−2 | nm2 mol−1 | ΠCf mN m−1 | kJ mol−1 |
|---|---|---|---|---|---|
| 0 | 0.005 | 0.30 | 5.53 | 37.9 | 183.4 |
| 0.0075 | 0.32 | 5.12 | 37.6 | 169.7 | |
| 0.01 | 0.27 | 6.15 | 33.6 | 174.0 | |
| 5 | 0.005 | 0.28 | 5.93 | 15.9 | 110.7 |
| 0.0075 | 0.27 | 6.15 | 16.8 | 114.1 | |
| 0.01 | 0.26 | 6.39 | 15.8 | 110.1 | |
| 7 | 0.005 | 0.25 | 6.64 | 12.4 | 102.1 |
| 0.0075 | 0.23 | 7.22 | 13.0 | 107.7 | |
| 0.01 | 0.24 | 6.92 | 12.0 | 96.2 | |
| 10 | 0.005 | 0.23 | 7.21 | 8.9 | 90.1 |
| 0.0075 | 0.23 | 7.21 | 8.8 | 87.6 | |
| 0.01 | 0.21 | 7.91 | 9.4 | 90.1 |
At all concentrations of NaCMC, the Cf values are higher than the cmc values in absence of polymer, signifying higher propensity towards micellization of the surfactant with polymer molecules. With increasing molecular weight of the polymer, the Cf values increase. This might be due to the availability of more and more reactive binding sites present in the polymer to the surfactant monomers.48 Thus, more amount of surfactant is required to bind the polymer. After Cs, coacervation starts and the system becomes a colloidal solution.47
Beyond Cf, free micelle formation starts with a fair degree of counter ion binding. At higher concentration of GS, the ion transport is obstructed e.g. colloidal coacervatate.47 Owing to this reason, the slope of the post Cf region decreases. The degree of ionization α1 and α2 both increase with increasing concentration of polymer, α1 decreases where as α2 increases with increasing molecular weight of the polymer. The standard Gibbs free energy changes for the micellization process at Cf were evaluated from eqn (3) and presented in Tables 2 and S2.†
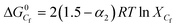 | (3) |
 was calculated from the eqn (4) and presented in Tables 2 and S2.†
was calculated from the eqn (4) and presented in Tables 2 and S2.†
 | (4) |
The turbidimetry showed almost sigmoidal pattern at all NaCMC concentrations. Similar type of turbidimetric profile was obtained in case of inulin-octadecyl trimethyl ammonium bromide system.47 There were three inflection points, T1, T2 and T3 in the turbidimetric profile (Fig. 3, inset). For GS-NaCMC system, the solution remains clear upto [GS] = T1. Beyond T1, the turbidity is clearly visible and increases sharply. The values were listed in Table 3. The values of T1 and T3 are very close to Cs and Cf respectively in the tensiometric profile. The inflection point T2 corresponds to the completion of adsorption of surfactants. T2 is quite lower than Cf value obtained tensiometrically. The turbidity of the solution slightly increases beyond T2 and then leveled off at T3 ≈ Cf. T3 values increase with increasing concentration of polymer and also with increasing concentration of IP. The turbidity slightly reduced beyond T3. There are reports of vanishing of visible turbidity due to the formation of free micelles which solubilise the coacervates.8,49 In our investigation, the coacervates are only partially solubilised even at higher concentration of polymer; but with increasing concentration of IP, turbidity decreases slightly i.e., the coacervates are partially soluble also in IP. From the solution, a small amount of the GS-NaCMC interacted complex was separated after standing for a long time.
| % of IP | [NaCMC]/g% | NaCMC-1 | NaCMC-2 | ||||
|---|---|---|---|---|---|---|---|
| T1 (mM) | T2 (mM) | T3 (mM) | T1 (mM) | T2 (mM) | T3 (mM) | ||
| 0 | 0.005 | 0.059 | 0.110 | 0.263 | 0.070 | 0.086 | 0.233 |
| 0.0075 | 0.076 | 0.147 | 0.307 | 0.071 | 0.136 | 0.277 | |
| 0.01 | 0.084 | 0.178 | 0.314 | 0.086 | 0.141 | 0.293 | |
| 5 | 0.005 | 0.062 | 0.114 | 0.290 | 0.062 | 0.091 | 0.237 |
| 0.0075 | 0.082 | 0.146 | 0.317 | 0.075 | 0.164 | 0.295 | |
| 0.01 | 0.094 | 0.184 | 0.328 | 0.107 | 0.185 | 0.350 | |
| 7 | 0.005 | 0.076 | 0.110 | 0.307 | 0.062 | 0.092 | 0.260 |
| 0.0075 | 0.090 | 0.145 | 0.324 | 0.107 | 0.176 | 0.314 | |
| 0.01 | 0.104 | 0.181 | 0.367 | 0.114 | 0.185 | 0.362 | |
| 10 | 0.005 | 0.072 | 0.112 | 0.328 | 0.070 | 0.105 | 0.275 |
| 0.0075 | 0.094 | 0.159 | 0.354 | 0.080 | 0.180 | 0.329 | |
| 0.01 | 0.120 | 0.182 | 0.447 | 0.118 | 0.193 | 0.420 | |
 | (5) |
 | (6) |
 and
and  against C. The intrinsic viscosity values obtained from these two equations were very close and enlisted in Table 4.
against C. The intrinsic viscosity values obtained from these two equations were very close and enlisted in Table 4.
| % of IP | [η]H/L g−1 | [η]M/L g−1 | kH | kM | VE | ν |
|---|---|---|---|---|---|---|
| 0 | 3.10 | 3.18 | 0.57 | −0.84 | 1.23 | 2.52 |
| (0.96) | (0.94) | (−0.41) | (−0.59) | (0.38) | (2.52) | |
| 5 | 2.90 | 2.94 | 0.47 | −0.89 | 1.14 | 2.55 |
| (0.89) | (0.87) | (−0.45) | (−0.54) | (0.35) | (2.53) | |
| 7 | 2.75 | 2.76 | 0.45 | −0.82 | 1.07 | 2.57 |
| (0.84) | (0.82) | (−0.42) | (-0.47) | (0.33) | (2.50) | |
| 10 | 2.50 | 2.54 | 0.52 | −0.56 | 0.99 | 2.52 |
| (0.78) | (0.77) | (−0.27) | (−0.37) | (0.31) | (2.51) |
The profile of intrinsic viscosity of NaCMC-1 and NaCMC-2 are shown in Fig. 4(a) and (b). There is a gradual decrease in [η]H with increase in concentration of IP. As the concentration of the ions in the solutions increases, counter ion binding onto the polyion chain is enhanced considerably. This causes the coiling of macromolecules which is reflected in the decrease in the intrinsic viscosity of the polyelectrolyte solution.52
 | ||
| Fig. 4 Profile of intrinsic viscosity of (a) NaCMC-1 and (b) NaCMC-2. (c) Profile of Y vs. C for the determination of Simha shape factor of NaCMC-1 and NaCMC-2 (Inset). | ||
The molecular weight of the polymer can be determined from Mark–Houwink equation
| [η]H = KMwa | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 208
000 and 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 of NaCMC in aqueous medium are 3.1 and 0.96 L g−1 respectively. There is a fair agreement between the calculated values with the observed values. In good solvent, K value is ∼0.35 for flexible and globular polymer.54 Huggins constant (kH) for NaCMC-1 is positive, while the value is negative for NaCMC-2 (in the parenthesis). Higher values of kH suggested some non-globular configuration of the polymer.
000 of NaCMC in aqueous medium are 3.1 and 0.96 L g−1 respectively. There is a fair agreement between the calculated values with the observed values. In good solvent, K value is ∼0.35 for flexible and globular polymer.54 Huggins constant (kH) for NaCMC-1 is positive, while the value is negative for NaCMC-2 (in the parenthesis). Higher values of kH suggested some non-globular configuration of the polymer.
The voluminosity (VE) of a polymer solution was calculated from the relative viscosity data at different concentrations of polymer (C) at a given temperature55–57 by using the following equation
 | (8) |
VE is determined from the intercept of the plot where Y is plotted against C. Voluminosity is the measure of volume of the solvated polymer molecules. Using the value of voluminosity and intrinsic viscosity, we can determine the shape factor (ν) from Simha equation58
| [η]H = VEν | (9) |
The Simha shape factor gives an idea about the shape of the polymer in the solution.59 The values of VE and ν were given in Table 4. The plots were shown in Fig. 4. Lower values of ν proposed spherical configuration of the polymer.55,60 In our study, the obtained values of shape factor are ∼2.5. Such low value of ν indicates the spherical configuration of the polymer.
Under similar condition, pure NaCMC-2 shows the isolated clusters and later fern-like aggregates (Fig. S2,† E′/F′).
4. Conclusion
The interaction of GS with NaCMC of two different molecular weights shows that NaCMC of higher molecular weight interacts strongly than NaCMC of lower molecular one. Like normal findings, the cac (origin of small polymer induced micelles), binding of the small aggregates with the polymer that maximizes at Cs and formation of free micelle in the solution at Cf were evaluated. With increasing concentration of polymer, Cs and Cf increase. With increasing the degree of substitution (S), the cac value decreases and distance between cac and Cs increases. The values of cac, Cs and Cf are dependent on solvent composition and increased with increasing composition of IP content. Coacervation is observed in the system without any additives. They do not dissolve even at higher concentration of surfactant. Formation of coacervation decreased with increasing concentration of IP. The nature and dimension of the NaCMC-GS interacted complex can be determined from viscosity, turbidity, DLS, SEM and TEM measurements. Spherical configuration of the polymer was obtained from viscosity measurement. The SEM measurements produce differences between the morphology of NaCMC and NaCMC-GS interacted complexes in water and IP–water media.Acknowledgements
S. D. and S. M. thank CSIR and UGC, Government of India, for Senior Research Fellowship. We also thank Prof. S. C. Bhattacharya, Department of Chemistry, Jadavpur University and Mr S. C. Mycap, Bose Institute, for DLS and SEM measurements, respectively.References
- A. J. Kirby, P. Camilleri, J. B. F. N. Engberts, M. C. Feiters, R. J. M. Nolte, O. Söderman, M. Bergsma, P. C. Bell, M. L. Fielden, C. L. G. Rodŕıguez, P. Guédat, A. Kremer, C. McGregor, C. Perrin, G. Ronsin and M. C. P. van Eijk, Angew. Chem., Int. Ed., 2003, 42, 1448 CrossRef CAS PubMed.
- A. Bhaumik and T. Tatsumi, Catal. Lett., 2000, 66, 181 CrossRef CAS.
- D. Chandra and A. Bhaumik, Ind. Eng. Chem. Res., 2006, 45, 4879 CrossRef CAS.
- D. Chandra, S. K. Das and A. Bhaumik, Microporous Mesoporous Mater., 2010, 128, 34 CrossRef CAS.
- C. Borde, V. Nardello, L. Wattebled, A. Laschewsky and J.-M. Aubry, J. Phys. Org. Chem., 2008, 21, 652 CrossRef CAS.
- H. B. Bohidar, J. Surf. Sci. Technol., 2008, 24, 105 CAS.
- E. D. Goddard, Colloids Surf., 1986, 19, 301 CrossRef CAS.
- Y. Li, P. L. Dubin, H. A. Havel, S. L. Edwards and H. Dautzenberg, Langmuir, 1995, 11, 2486 CrossRef CAS.
- Y. Wang, K. Kimura and P. L. Dubin, Macromolecules, 2000, 33, 3324 CrossRef CAS.
- T. Chakraborty, I. Chakraborty and S. Ghosh, Langmuir, 2006, 22, 9905 CrossRef CAS PubMed.
- Y. Pi, Y. Shang, H. Liu, Y. Hu and J. Jiang, J. Colloid Interface Sci., 2007, 306, 405 CrossRef CAS PubMed.
- C. D. Bain, P. M. Claesson, D. Langevin, R. Meszaros, T. Nylander, C. Stubenrauch, S. Titmuss and R. V. Klitzing, Adv. Colloid Interface Sci., 2010, 155, 32 CrossRef CAS PubMed.
- S. Mukherjee, A. Dan, S. C. Bhattacharya, A. K. Panda and S. P. Moulik, Langmuir, 2011, 27, 5222 CrossRef CAS PubMed.
- S. M. Saleem and A. Hernandez, J. Surf. Sci. Technol., 1987, 3, 1 CAS.
- D. Ali, S. Bolton and G. Gaylord, J. Appl. Polym. Sci., 1991, 42, 947 CrossRef.
- A. Bhattacharyya and J. F. Argillier, J. Surf. Sci. Technol., 2005, 21, 161 CAS.
- B. Jönsson, B. Lindman, K. Holmberg and B. Kronberg, Surfactants and Polymers in Aqueous Solution, John Wiley & Sons Ltd., West Sussex, UK, 1998 Search PubMed.
- S. Ghosh and S. P. Moulik, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 1999, 38, 10 Search PubMed.
- S. Ghosh and S. P. Moulik, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 1999, 38, 201 Search PubMed.
- A. Chilkoti, T. Christensen and J. A. Mackay, Curr. Opin. Chem. Biol., 2006, 10, 652 CrossRef CAS PubMed.
- Y. Yeo, E. Bellas, W. Firestone, R. Langer and D. S. Kohane, J. Agric. Food Chem., 2005, 53, 7518 CrossRef CAS PubMed.
- S. Ghosh and S. P. Moulik, J. Surf. Sci. Technol., 1998, 14, 110 CAS.
- P. Ilekti, L. Piculell, F. Torunilhac and B. Cabane, J. Phys. Chem. B, 1999, 103, 9831 CrossRef CAS.
- A. Das Burman, S. Ghosh and A. R. Das, J. Nanofluids, 2014, 3, 140 CrossRef.
- S. Mondal, S. Das and S. Ghosh, J. Surfactants Deterg., 2015, 18, 471 CrossRef CAS.
- H. Wang and Y. Wang, J. Phys. Chem.B, 2010, 114, 10409 CrossRef CAS PubMed.
- A. Vanerek and T. G. M. van de Ven, Colloids Surf., A, 2006, 273, 55 CrossRef CAS.
- S. Das, I. Mukherjee, B. K. Paul and S. Ghosh, Langmuir, 2014, 30, 12483 CrossRef CAS PubMed.
- B. Naskar, A. Dan, S. Ghosh and S. P. Moulik, J. Chem. Eng. Data, 2010, 55, 2424 CrossRef CAS.
- B. Mandal, S. P. Moulik and S. Ghosh, J Surface Interfac Mater, 2013, 1, 77 CrossRef.
- E. K. Just and T. G. Majewicz, Encyclopedia of Polymer Science and Engineering, John Wiley and Sons, New York, 1985, vol. 3, p. 226 Search PubMed.
- S. Guillot, M. Delsanti, S. Désert and D. Langevin, Langmuir, 2003, 19, 230 CrossRef CAS.
- J. Mata, J. Patel, N. Jain, G. Ghosh and P. Bahadur, J. Colloid Interface Sci., 2006, 297, 797 CrossRef CAS PubMed.
- S. Trabelsi, E. Raspaud and D. Langevin, Langmuir, 2007, 23, 10053 CrossRef CAS PubMed.
- N. Sardar, M. Kamil and Kabir-ud-Din, Colloids Surf., A, 2012, 415, 413 CrossRef CAS.
- F. M. Menger and J. S. Keiper, Angew. Chem., Int. Ed., 2000, 39, 1906 CrossRef.
- K. Tsubone and S. Ghosh, J. Surfactants Deterg., 2003, 6, 225 CrossRef CAS.
- R. Zana and J. Xia, Gemini Surfactants: Synthesis, Interfacial and Solution-phase Behavior and Applications, Marcel Dekker, Inc., New York, 2004 Search PubMed.
- Y. Han and Y. Wang, Phys. Chem. Chem. Phys., 2011, 13, 1939 RSC.
- F. M. Menger, J. S. Keiper and V. Azov, Langmuir, 2000, 16, 2062 CrossRef CAS.
- S. Ghosh, J. Colloid Interface Sci., 2001, 244, 128 CrossRef CAS.
- S. Das, S. Maiti and S. Ghosh, RSC Adv., 2014, 4, 12275 RSC.
- S. Das, B. Naskar and S. Ghosh, Soft Matter, 2014, 10, 2863 RSC.
- M. J. Schwuger and H. Lange, Tenside, 1968, 5, 257 CAS.
- X. Wang, Y. Li, J. Li, J. Wang, Y. Wang, Z. Guo and H. Yan, J. Phys. Chem. B, 2005, 109, 10807 CrossRef CAS PubMed.
- C. Wang and K. C. Tam, Langmuir, 2002, 18, 6484 CrossRef CAS.
- A. Dan, S. Ghosh and S. P. Moulik, J. Phys. Chem. B, 2009, 113, 8505 CrossRef CAS PubMed.
- A. P. Romani, M. H. Gehlen and I. Rosangela, Langmuir, 2005, 21, 127 CrossRef CAS PubMed.
- M. Lundin, L. Macakova, A. Dedinaite and P. Claesson, Langmuir, 2008, 24, 3814 CrossRef CAS PubMed.
- M. L. Huggins, J. Am. Chem. Soc., 1942, 64, 2716 CrossRef CAS.
- E. O. Kraemer, Ind. Eng. Chem., 1938, 30, 1200 CrossRef CAS.
- R. Sharma, B. Das, P. Nandi and C. Das, J. Polym. Sci., Part B, 2010, 48, 1196 CrossRef CAS.
- T. E. Eremeeva and T. O. Bykova, Carbohydr. Polym., 1998, 36, 319 CrossRef CAS.
- C. Tanford, Physical Chemistry of Macromolecules, John Wiley and Sons, New York, 1961, p. 391 Search PubMed.
- S. Ghosh, Colloid Surface Physicochem Eng Aspect, 2005, 264, 6 CrossRef CAS.
- A. Rangaraj, V. Vangani and A. K. Rakshit, J. Appl. Polym. Sci., 1997, 66, 45 CrossRef CAS.
- V. Vangani and A. K. Rakshit, J. Appl. Polym. Sci., 1996, 60, 1005 CrossRef CAS.
- R. Simha, J. Phys. Chem., 1940, 44, 25 CrossRef CAS.
- H.-H. Kohler and J. Strnad, J. Phys. Chem., 1990, 94, 7628 CrossRef CAS.
- A. S. Narang and U. C. Garg, J. Indian Chem. Soc., 1989, 66, 214 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The interfacial parameters for micellization and interaction characteristics of 14-4-14 with NaCMC-2 in aqueous and aqueous-IP medium obtained from conductometry, tensiometry and DLS methods, structure of NaCMC and particle size distribution and SEM images have been provided. This material is available free of charge via the internet. See DOI: 10.1039/c6ra00640j |
| This journal is © The Royal Society of Chemistry 2016 |









