Highly efficient regio and diastereoselective synthesis of functionalized bis-spirooxindoles and their antibacterial properties†
Pambala Ramesha,
Kadiyala Srinivasa Raoa,
Rajiv Trivedi*a,
B. Sudheer Kumarb,
R. S. Prakashamb and
B. Sridharc
aInorganic and Physical Chemistry Division, CSIR-Indian Institute of Chemical Technology, Hyderabad 500007, India. E-mail: rtrajiv401@gmail.com; Fax: +91-40-27193275
bBio Engineering and Environmental Centre, CSIR-Indian Institute of Chemical Technology, Hyderabad 500007, India
cLaboratory of X-ray Crystallography, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, India
First published on 4th March 2016
Abstract
A simple, efficient, regioselective and diastereoselective method has been developed for the synthesis of diversely functionalized spirooxindole-pyrrolidines using 0.5 mol% of ceric ammonium nitrate in aqueous medium. All the new compounds are tested for in vitro antimicrobial activity. All the synthesized products have shown good antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus (Gram-positive organisms), Salmonella paratyphi, Pseudomonas aeruginosa and Salmonella typhi (Gram-negative organism).
Introduction
Fused polycyclic compounds such as spirocyclic oxindoles have emerged as elegant synthetic targets due to their prevalence in numerous natural products and biologically active molecules (Fig. 1).1 The spiro-ring fused at the C3 position of the oxindole constitutes the key structural feature of such compounds.2 Several groups have been involved in developing atom-economic and eco-friendly routes for the synthesis of such spirooxindole derivatives.3 For instance, recently, Luo and co-workers have reported the 1,3-dipolar cycloadditions of isatins, benzylamine and benzylideneacetones to prepare a series of novel spiropyrrolidine-oxindoles.4One of the latest trends in the realm of organic and medicinal chemistry encompasses the proficiency of multi-component reactions to synthesize biologically relevant complex molecules. These single step reactions embody the advantages of having high atom economy rates, excellent bond-forming efficiency leading to a wide structural diversities.5,6 One such reactions, namely, the 1,3-dipolar cycloaddition reaction have been used extensively for the synthesis of highly functionalized cyclic organic compounds of biological interest.7 Substituted pyrrolidines having multiple stereocenters are synthesized from dipoles such as azomethine ylides involving such cycloaddition reactions.8,9 In recent times, multicomponent reactions performed in aqueous media have become much sought after methodology.10 Water as a medium has economic and environmental advantages.11 Moreover, unique selectivity and reactivity can be accomplished in aqueous media as compared to organic solvents.12 Enhanced rate of reaction as well as stability of reaction intermediates are some of the additional features associated with the aqueous media.13
Over the past couple of years, our group has been involved in the design and development of different types of heterocyclic compounds using Ce based heterogeneous catalysts under solvent-free conditions or using aqueous media.14 Based on these results we envisaged that the use of Lewis acid such as Ceric Ammonium Nitrate (CAN) can be investigated for the formation of the desired spirooxindoles. CAN has certain advantages such as low toxicity, inexpensive, reasonably soluble in many organic media, air stable and can be handled easily.15 To the best of our knowledge, the cycloaddition reaction of isatin, benzyl amine and (Z)-3-(2-oxo-2-phenylethylidene)indolin-2-one is not yet reported. Herein, we report the Ceric Ammonium Nitrate (CAN) catalyzed efficient regioselective and diastereoselective 1,3-dipolar cycloaddition reaction of isatin, benzyl amine and (Z)-3-(2-oxo-2-phenylethylidene)indolin-2-one in water to produce novel functionalized spirooxindole-pyrrolidines in good yield. These compounds are also screened for their antibacterial activities.
Results and discussion
Isatin (3a), benzyl amine (2a) and an exocyclic enone derived from isatin (3a) were selected as model substrates. The model reaction was performed in water at 100 °C to give a poor yield (10%) of the desired product after 12 h. This reaction was then performed in the presence of 1 mol% of ceric ammonium nitrate under identical conditions and to our delight, the reaction proceeded smoothly and desired product was obtained in 90% yield after 2 h. The product was characterized by spectroscopic data (NMR, Mass, IR and HRMS). The structure and stereochemistry of spirooxindole 4s is unambiguously confirmed by single crystal X-ray crystallographic analysis (Fig. 2).17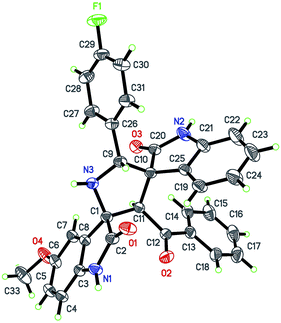 | ||
| Fig. 2 X-ray crystal structure of 4s (ORTEP diagram).17 | ||
Furthermore, to examine the effect of the amine substrates, the three component reaction was also conducted using other amines such as ethyl amine and allyl amine. It was found that the reaction was sluggish and no formation of the desired product was observed under identical reaction conditions. The model reaction was then conducted in different solvents such as methanol, ethanol, dichloromethane, tetrahydrofuran, toluene and acetonitrile in presence of 1 mol% of ceric ammonium nitrate under identical conditions. The results are summarized in Table 1. It was observed that the reaction conducted in the presence of 0.5 mol% of CAN in water at 100 °C in 2 h gave the best result. Further increasing or decreasing the reaction temperature as well as the mol ratio of the catalyst had no significant improvement on the yield and efficiency of the reaction.
| Entry | Solvent | Catalyst | Timeb (h) | Yieldc (%) |
|---|---|---|---|---|
| a All reactions were carried out with 1 (1 mmol), 2 (1 mmol) and 3 (1 mmol), solvent (3 ml), CAN 1 mol%.b Reaction time.c Yields of isolated product.d CAN 0.1 mol%.e CAN 0.5 mol%.f CAN 0.7 mol%. | ||||
| 1 | Methanol | CAN | 8 | 50 |
| 2 | Ethanol | CAN | 8 | 60 |
| 3 | DCM | CAN | 7 | 70 |
| 4 | Chloroform | CAN | 7 | 60 |
| 5 | Toluene | CAN | 10 | 40 |
| 6 | THF | CAN | 10 | 50 |
| 7 | Water | CAN | 2 | 90 |
| 8 | CH3CN | CAN | 12 | 70 |
| 9 | Water | — | 12 | 10 |
| 10 | Water | CAN | 2 | 70d |
| 11 | Water | CAN | 2 | 90e |
| 12 | Water | CAN | 2 | 90f |
The electronic effect of the substituents present on isatin and benzylamine was also examined under the optimized conditions for the cycloaddition reaction. It was observed that cycloaddition reactions with isatins having substituents such as NO2, OMe and Me at C(5) position were slow and the desired products were obtained in moderate yields (entries 4e, 4f, 4g, 4l, 4m, 4n, 4s, 4l, Table 2). Whereas, the cycloaddition reaction of isatins with halogen substitutents (Cl, Br and I) at C(5) (entries 4b, 4c, 4d, 4i, 4j, 4k, 4p, 4q and 4r Table 2) proceeded smoothly to give the corresponding spirooxindoles in high yield. The cycloaddition reaction of simple isatin (entries 4a, 4h and 4o) and 5-iodo isatin (entries 4d, 4k and 4r) gave the required spirooxindoles in good yield. The effect of substituents present on the benzylamine was also sequentially examined. It was noticed that cycloaddition reaction of the methoxy substituted benzylamines (entries 4h–4n) was slow and gave the desired spirooxindoles in low yield. On the other hand simple (entries 4a–4g) and fluoro (entries 4o–4t) substituted benzylamines participated in cycloaddition reaction smoothly and gave the corresponding spirooxindoles. All the spirooxindole derivatives were completely characterized using spectroscopic analysis. The configuration of the compounds in Table 2 was assigned based on the single crystal X-ray structure of 4s.
Based on the experimental results as described above, a plausible mechanism could be proposed as shown in Scheme 1. Initially benzylamine B reacts with isatin A to form the azomethine ylide intermediate C in the presence of CAN. This azomethine ylide acts as a dipole which readily reacts with available dipolarophile D to give diversely functionalized regioselective spirooxindoles.
All the synthesized spirooxindoles were screened for their in vitro antimicrobial activity against the bacterial strains Bacillus subtilis, Staphylococcus aureus, and Micrococcus luteus (Gram-positive), Salmonella paratyphi, Pseudomonas aeruginosa, Salmonella typhi, (Gram-negative) and Candida albicans (Candidal infections) by Agar Well Diffusion Method.
Naturally occurring spirooxindoles (horsfiline, elacomine, alstonisine, and mitraphylline etc.) generally show significant biological applications.16 Analysis of the synthesized twenty spirooxindole derivatives for their antimicrobial activities against six bacterial and one Candida species by well plate method revealed that all derivatives exhibited moderate to good antimicrobial activity against the tested organisms (Table 3). It is interesting to note that all derivatives were more active against Gram positive bacteria as compared to the Gram negative and Candida albicans. Among all derivatives, halogenated (4b, 4c and 4d), methoxy halogenated (4g and 4h) and di-halogenated derivatives showed significant antibacterial activity against Micrococcus luteus. However, derivatives such as 4b, 4c, 4k and 4l lost their antimicrobial activity against Gram negative bacteria (S. typhi and P. aeruginosa) indicating the imperative role of cell wall composition as well as the genetic nature of the organism play significant role in conferring the antibiotic nature of these compounds. Surprisingly, all the derivatives denoted moderate growth inhibitory activity against C. albicans. Similar studies were reported by Bhaskar et al., while studying on spirooxindole derivatives.16
| Compound | P. aeruginosa | S. typhi | S. paratyphi | B. subtilis | S. aureus | M. luteus | C. albicans |
|---|---|---|---|---|---|---|---|
| a Standard: for bacteria: streptomycin sulphate (1 mg ml−1). For Candida: fluconazole (1 mg ml−1) sample loaded in each well: 100 μl. Test sample conc.: 1 mg ml−1, readings in mm (millimetres) experiment results are in duplicates. | |||||||
| 4a | 12 | 0 | 13 | 13 | 15 | 16 | 12 |
| 4b | 0 | 12 | 15 | 20 | 18 | 23 | 17 |
| 4c | 0 | 0 | 11 | 18 | 20 | 15 | 13 |
| 4d | 13 | 15 | 15 | 18 | 15 | 21 | 11 |
| 4e | 13 | 0 | 14 | 16 | 17 | 17 | 12 |
| 4f | 13 | 17 | 10 | 18 | 16 | 17 | 14 |
| 4g | 20 | 14 | 12 | 16 | 20 | 21 | 17 |
| 4h | 16 | 0 | 16 | 16 | 20 | 23 | 15 |
| 4i | 20 | 13 | 16 | 16 | 12 | 23 | 11 |
| 4j | 18 | 14 | 13 | 15 | 16 | 19 | 13 |
| 4k | 0 | 0 | 14 | 14 | 14 | 21 | 14 |
| 4l | 0 | 0 | 12 | 0 | 16 | 13 | 11 |
| 4m | 18 | 14 | 14 | 18 | 13 | 21 | 12 |
| 4n | 17 | 18 | 14 | 18 | 17 | 22 | 15 |
| 4o | 17 | 15 | 13 | 15 | 18 | 20 | 13 |
| 4p | 20 | 18 | 14 | 20 | 15 | 22 | 14 |
| 4q | 20 | 18 | 15 | 18 | 14 | 26 | 14 |
| 4r | 20 | 16 | 11 | 18 | 17 | 19 | 15 |
| 4s | 18 | 12 | 12 | 18 | 16 | 18 | 12 |
| 4t | 19 | 15 | 15 | 18 | 0 | 17 | 12 |
| Std | 30 | 25 | 23 | 24 | 20 | 26 | 0 |
Conclusions
In summary, we have developed a simple, efficient, regioselective and diastereoselective approach for the synthesis of diversely functionalized spirooxindoles-pyrrolidines in presence of catalytic amount of ceric ammonium nitrate in aqueous medium. In addition to this, we have screened all the new compounds for in vitro antimicrobial activity. All the synthesized products shown good antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, and Micrococcus luteus (Gram-positive organisms), Salmonella paratyphi, Pseudomonas aeruginosa, Salmonella typhi, (Gram-negative organisms) and Candida albicans (Candidal infections). The result shows that these spirooxindole derivatives will be suitable for further explorations in this field for research of potent antimicrobial agents.Experimental section
General information
Benzylamine, isatins, acetophenone, ceric ammonium nitrate and all solvents were purchased from Sigma Aldrich and Alfa Aesar company and used without further purification as received. All 1H and 13C NMR spectra were recorded in deuterated chloroform (CDCl3) or CDCl3 + DMSO-d6 (deuterated dimethyl sulfoxide) (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) on Avance 300 or Avance 400 or Avance 500 spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) relative to residual CHCl3 (1H: δ 7.26 ppm, 13C: δ 77.00 ppm) as an internal reference. Coupling constants (J) are reported in Hertz (Hz). Peak multiplicity is indicated as follows: s—singlet, d—doublet, t—triplet, q—quartet, m—multiplet and dd—doublet of doublet. Melting points were measured on a BUCHI melting point machine. IR spectra were recorded on Thermo Nicolet FT/IR-5700 spectrometer. Mass spectra were recorded with a Waters 2695 for low (MS) and Thermo Scientific Exactive spectrometer for HRMS. HRMS data were obtained using ESI method.
4) on Avance 300 or Avance 400 or Avance 500 spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) relative to residual CHCl3 (1H: δ 7.26 ppm, 13C: δ 77.00 ppm) as an internal reference. Coupling constants (J) are reported in Hertz (Hz). Peak multiplicity is indicated as follows: s—singlet, d—doublet, t—triplet, q—quartet, m—multiplet and dd—doublet of doublet. Melting points were measured on a BUCHI melting point machine. IR spectra were recorded on Thermo Nicolet FT/IR-5700 spectrometer. Mass spectra were recorded with a Waters 2695 for low (MS) and Thermo Scientific Exactive spectrometer for HRMS. HRMS data were obtained using ESI method.
General experimental procedure
A 5 ml RB flask containing isatin (1) (1.0 mmol), benzylamine (2) (1.0 mmol), isatin chalcone (3) (1.0 mmol), ceric ammonium nitrate (0.5 mol%) and water (2 ml) was placed in oil bath and refluxed for the appropriate time, at 100 °C (temperature monitored by a thermometer). The progress of reaction was monitored by TLC. After the reaction was completed, the aqueous mixture was extracted with ethyl acetate and dried over Na2SO4, and the solvent was removed under reduced pressure. The reaction mixture was purified by (silica gel) column chromatography (hexane/AcOEt, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 as eluent) to give pure products.
20 as eluent) to give pure products.
Antimicrobial activity
Nutrient agar (1.3%) was used for growth of bacteria, YPD (yeast extract 1%, peptone and dextrose 2%) agar for Candida. Test samples, standards (streptomycin sulphate for bacteria and fluconazole for Candida) were dissolved in DMSO (control) at a concentration of 1 mg ml−1. Initially nutrient agar, YPD agar and Petri dishes were autoclaved at 121 °C for 20 min at 15lb pressure, separately. After sterilization, media was poured in Petri plates and allowed to solidification. Later, active cultures were spread on solidified plates by using sterile cotton swabs. Wells (8 mm) are made with sterile cork borer and each well is filled with 100 μl of subsequent test samples and standard. Initially plates were placed at 4 °C for proper diffusion of sample in to agar medium, later the plates were incubated at 37 °C for growth of cultures. After incubation, zone of growth inhibition was measured with the help of calibrated scale. Experiment was duplicated to minimize the errors.Acknowledgements
This work is financially supported by the in-house project MLP-0007 and XIIth Five Year Plan project CSC-0132 of CSIR-IICT. P. R is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi for financial support from the XIIth Five Year Plan Project SURE.Notes and references
- (a) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Org. Biomol. Chem., 2012, 10, 5165 RSC; (b) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed; (c) L. Hong and R. Wang, Adv. Synth. Catal., 2013, 355, 1023 CrossRef CAS.
- (a) J. Li, J. Wang, Z. Xu and S. Zhu, ACS Comb. Sci., 2014, 16, 506 CrossRef CAS PubMed; (b) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, J. Med. Chem., 2008, 51, 5731 CrossRef CAS PubMed.
- (a) M. A. Ali, R. Ismail, T. S. Choon, Y. K. Yoon, A. C. Wei, S. Pandian, R. S. Kumar, H. Osman and E. Manogaran, Bioorg. Med. Chem. Lett., 2010, 20, 7064 CrossRef CAS PubMed; (b) K. Revathy and A. Lalitha, RSC Adv., 2014, 4, 279 RSC; (c) J. Li, J. Wang, Z. Xu and S. Zhu, ACS Comb. Sci., 2014, 16, 506 CrossRef CAS PubMed; (d) S. Kanchithalaivan, R. V. Sumesh and R. R. Kumar, ACS Comb. Sci., 2014, 16, 566 CrossRef CAS PubMed; (e) F. Rouatbi, M. Askri, F. Nana, G. Kirsch, D. Sriram and P. Yogeeswari, Tetrahedron Lett., 2016, 57, 163 CrossRef CAS; (f) J.-A. Xiao, H.-G. Zhang, S. Liang, J.-W. Ren, H. Yang and X.-Q. Chen, J. Org. Chem., 2013, 78, 11577 CrossRef CAS PubMed; (g) A. I. Almansour, N. Arumugam, R. S. Kumar, G. Periyasami, H. A. Ghabbour and H.-K. Fun, Molecules, 2015, 20, 780 CrossRef PubMed.
- C. Peng, J. Ren, J. Xiao, H. Zhang, H. Yang and Y. Luo, Beilstein J. Org. Chem., 2014, 10, 352 CrossRef PubMed.
- (a) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed; (b) L. F. Tietze and G. Kettschau, Top. Curr. Chem., 1997, 189, 1 CrossRef CAS; (c) L. F. Tietze and U. Beifuss, Comprehensive Organic Synthesis, ed. B. M. Trost, 1991, vol. 2, p. 341 Search PubMed.
- (a) J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300 CrossRef CAS PubMed; (b) D. Tejedor and F. Garcia-Tellado, Chem. Soc. Rev., 2007, 36, 484 RSC; (c) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Methen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899 CrossRef CAS PubMed; (d) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef.
- (a) K. V. Gothelf and K. A. Jørgensen, Chem. Rev., 1998, 98, 863 CrossRef CAS PubMed; (b) G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484 CrossRef CAS PubMed; (c) L. M. Stanley and M. P. Sibi, Chem. Rev., 2008, 108, 2887 CrossRef CAS PubMed.
- J. Adrio and J. C. Carretero, Chem. Commun., 2011, 47, 6784 RSC.
- (a) J. R. Hwu and K.-Y. King, Curr. Sci., 2001, 81, 1043 CAS; (b) V. Nair, S. B. Panicker, L. G. Nair, T. G. George and A. Augustine, Synlett, 2003, 156 CrossRef CAS; (c) V. Nair, L. Balagopal, R. Rajan and J. Mathew, Acc. Chem. Res., 2004, 37, 21 CrossRef CAS PubMed; (d) V. Nair and A. Deepthi, Chem. Rev., 2007, 107, 1862 CrossRef CAS PubMed; (e) V. Sridharan and J. C. Menendez, Chem. Rev., 2010, 110, 3805 CrossRef CAS PubMed.
- For recent reviews on organic reactions in water, see: Organic Reactions in Water, ed. U. M. Lindström, Blackwell, Oxford, UK, 2007 Search PubMed.
- (a) C.-J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed; (b) C.-J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC.
- (a) U. M. Lindström, Chem. Rev., 2002, 102, 2751 CrossRef; (b) S. Kobayashi, Pure Appl. Chem., 2007, 79, 235–245 CrossRef CAS.
- For domino reactions in water, see some selected examples, (a) K. Kumaravel and G. Vasuki, Green Chem., 2009, 11, 1945 RSC; (b) S.-J. Tu, Y. Zhang, H. Jiang, B. Jiang, J.-Y. Zhang, R.-H. Jia and F. Shi, Eur. J. Org. Chem., 2007, 1522 CrossRef CAS; (c) Z.-Y. Lu, C.-M. Hu, J.-J. Guo, J. Li, Y.-X. Cui and Y.-X. Jia, Org. Lett., 2010, 12, 480–483 CrossRef CAS PubMed; (d) G. Giorgi, S. Miranda, P. Lopez-Alvarado, C. Avendano, J. Rodriguez and J. C. Menendez, Org. Lett., 2005, 7, 2197 CrossRef CAS PubMed; (e) Y. Jung and R. A. Marcus, J. Am. Chem. Soc., 2007, 129, 5492 CrossRef CAS PubMed; (f) L. Chen and C.-J. Li, Green Chem., 2003, 5, 627 RSC; (g) J. Zhang and C.-J. Li, J. Org. Chem., 2002, 67, 3969 CrossRef CAS PubMed; (h) K. Kumaravel and G. Vasuki, Curr. Org. Chem., 2009, 13, 1820 CrossRef CAS; (i) N. S. Kumar, L. C. Rao, N. Jagadeesh Babu and H. M. Meshram, RSC Adv., 2015, 5, 95539 RSC; (j) J. K. Beattie, C. S. P. McErlean and C. B. W. Phippen, Chem.–Eur. J., 2010, 16, 8972 CrossRef CAS PubMed.
- (a) A. M. Akondi, M. L. Kantam, R. Trivedi, B. Sreedhar, S. K. Buddana, R. S. Prakasham and S. Bhargava, J. Mol. Catal. A: Chem., 2014, 386, 49 CrossRef CAS; (b) A. M. Akondi, M. L. Kantam, R. Trivedi, J. Bharatam, S. P. B. Vemulapalli, S. K. Bhargava, S. K. Buddana and R. S. Prakasham, J. Mol. Catal. A: Chem., 2016, 411, 325 CrossRef CAS.
- (a) T. L. Ho, Synthesis, 1973, 347 CrossRef CAS; (b) T. Imamoto, M. Nishiura, Y. Yamanoi, H. Tsuruta and K. Yamaguchi, Chem. Lett., 1996, 25, 875 CrossRef; (c) J. R. Hwu and K.-Y. King, Curr. Sci., 2001, 81, 1043 CAS; (d) V. Nair, S. B. Panicker, L. G. Nair, T. G. George and A. Augustine, Synlett, 2003, 156 CrossRef CAS; (e) V. Nair, L. Balagopal, R. Rajan and J. Mathew, Acc. Chem. Res., 2004, 37, 21 CrossRef CAS PubMed; (f) V. Nair and A. Deepthi, Chem. Rev., 2007, 107, 1862 CrossRef CAS PubMed; (g) V. Sridharan and J. C. Menendez, Chem. Rev., 2010, 110, 3805 CrossRef CAS PubMed.
- G. Bhaskar, Y. Arun, C. Balachandran, C. Saikumar and P. T. Perumal, Eur. J. Med. Chem., 2012, 51, 79 CrossRef CAS PubMed.
- CCDC number of the 4s is 1440318.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1440318. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra00613b |
| This journal is © The Royal Society of Chemistry 2016 |





