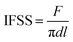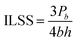Interfacially reinforced carbon fiber/epoxy composites by grafting melamine onto carbon fibers in supercritical methanol
Min Zhaoa,
Linghui Menga,
Lichun Maa,
Guangshun Wua,
Yuwei Wangab,
Fei Xiea and
Yudong Huang*a
aSchool of Chemical Engineering and Technology, Harbin Institute of Technology, Harbin 150001, China. E-mail: ydhuang.hit1@yahoo.com.cn; Fax: +86 451 86221048; Tel: +86 451 86414806
bCollege of Materials Science and Engineering, Qiqihar University, Qiqihar 161006, China
First published on 17th March 2016
Abstract
Melamine used as a coupling agent was functionalized onto a carbon fiber (CF) surface in supercritical methanol to improve the interfacial properties of CF reinforced epoxy composites. Fourier transform infrared spectroscopy (FTIR), Raman spectra and X-ray photo electron spectroscopy (XPS) confirmed the successful grafting of melamine molecules onto the fiber surface. Scanning electron microscopy (SEM) images showed that melamine was grafted onto the CF surface uniformly and the surface roughness was enhanced obviously. Dynamic contact angle analysis (DCA) revealed the significant improvement in the surface energy and wettability. Compared with the untreated CF composites, the interfacial shear strength (IFSS) and inter-laminar shear strength (ILSS) of composites after melamine modification increased by 41.3% and 36.4%, respectively. The impact properties were also improved significantly. In addition, the reinforcing and toughening mechanisms were also discussed. Meanwhile, supercritical treatment did not decrease the single filament tensile strength obviously.
1. Introduction
CF reinforced composites have been widely used in a variety of high-performance structural applications, such as in the aerospace, marine and automobile industries, owing to their excellent mechanical properties, low weight, chemical resistance and environmental stability.1,2 However, the potential of CF reinforced composites has not yet been fully exploited due to the chemically inert surface and low surface energy of the fiber, which make it difficult to exhibit desired interfacial adhesion with matrix resin.3,4 Therefore, numerous surface modifications have been developed to make the fiber surface more compatible with polymer matrix such as oxidation, coating, grafting and loading, etc.5–9 Among these efforts, grafting coupling agents onto CF surface is an effective method to enhance the interfacial strength of composites. Coupling agents can act as molecular bridges between fiber and matrix resin via chemical bonds. In general, the interfacial strength is greatly associated with the numbers of chemical bonds.1 However, most conventional coupling agents only have two reactive groups in each molecule,10,11 and novel coupling agents with more than two active functional groups which have been developed in recent years always need harsh reaction condition, such as long reaction time and in vacuum.12,13Melamine is a six membered heterocyclic aromatic organic compound. It possesses some unique polar characteristics with three amino groups and is widely used as nitrogen containing precursor to enrich carbons in electrochemical capacitor material.14 Recently, some researches about the immobilization of melamine at surface which are less widespread have been reported. Adam et al. reported its grafting on silica support via a spacer and the further use of the modified surface for catalysis.15 A. Grondein et al. grafted melamine on carbon Vulcan XC72R surface through the in situ diazotization to evaluate its potential as a CO2 solid sorbent.16 So far, there has no related report on surface treatment of CF with melamine. The presence of three amine functionalities in the melamine molecule could be useful for that purpose. Since the amino density of melamine is higher than conventional coupling agents, the chemically interact possibility between melamine functionalized CF and epoxy resin is supposed to be much higher, resulting in a high chemical bonding at the interface and improving the interfacial properties of composites.
To further improve the efficiency of functionalizing CF, supercritical fluids could be proposed as alternative reaction media, since the fluids are readily available, environmentally friendly and easy to handle.17,18 They possess unique properties intermediate of those between gases and liquids: low viscosities, a high mass transport co-efficient, high diffusivity, and a pressure dependent solvent power.19 Based on these properties, the coupling agent can transport more efficiently and distribute more homogeneously in supercritical fluids, which could shorten the reaction time and make supercritical fluids ideal candidates for reactions.20 Nowadays the application of supercritical fluids for CF have been developed. Supercritical acetone has been used for cleaning the surfaces of CF and show a good cleaning efficiency.21 Some studies have reported the recycling methods for CF with supercritical propanol and water.22,23 However, there is few research on the CF grafting in supercritical fluids.
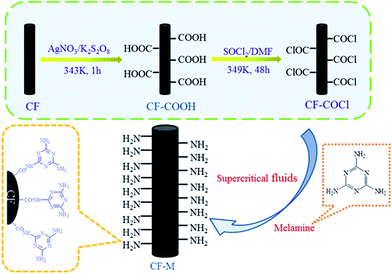
In this study, we firstly functionalize CF by grafting melamine in high-efficiency supercritical fluid, the process is illustrated in Fig. 1. Methanol is selected as the supercritical fluid for a better dissolution of melamine. Grafting melamine onto CF surface as coupling agents not only enhance the polarity of fiber surface but also provide more reactive sites for the reaction with epoxy groups of matrix, which can improve the interfacial adhesion between CF and matrix resin. Furthermore, the method in this paper is facile, effective and presents experimental feasibility which indicate that supercritical methanol might be a suitable medium for CF grafting and helpful for further improvement in adhesion property.
2. Materials and methods
2.1. Materials
PAN-based carbon fibers (diameter 7 μm and density 1.76 g cm−3) were purchased from Sinosteel Jilin Carbon Co., China. Melamine was received from Aladdin Co. All other chemicals (acetone, dimethyl formamide, methanol) obtained from Tianjin Bodi Organic Chemicals Co. Ltd. were reagent-grade.2.2. Surface modification of CF
CF were firstly extracted by Soxhlet with acetone to remove the polymer sizing and pollutants on the surface of CF, denoted as untreated CF. Subsequently, the CF were oxidized in AgNO3/K2S2O8 solution at 343 K for 1 h to obtain the carboxyl functionalized CF (CF-COOH).24 After being dried, the CF-COOH were reacted with 100 mL SOCl2 and 5 mL DMF at 349 K for 48 h to yield acyl chloride functionalized CF (CF-COCl).Accurately weighted melamine was poured into 200 mL volumetric flask, dissolved in the 50 mL methanol. Then the solution mentioned above and CF-COCl were transferred into a stainless steel autoclave with a high pressure valve. The autoclave was heated to 553 K and the treated time was varied from 5, 15, 25 to 35 min. After that, the autoclave was moved slowly into cold water and cooled down to the room temperature. The functionalized CF were washed with methanol several times to remove the unreacted melamine, and then dried at 393 K for 4 h. The functionalized CF denoted as CF-M-t, t = 5, 15, 25, 35.
2.3. Characterization
The FTIR characterization of the untreated and grafted CF was performed on FTIR spectrophotometer (Nicolet, Nexus670, USA). Before analysis, the carbon fiber was dried for 2 h at 423 K. The spectra were acquired by scanning the specimens for 64 times in the wavenumber range of 4000 to 500 cm−1.The surface structures of CF were analyzed by Raman spectrometer (Invia Renishaw 2000, UK) with an Ar+ laser (514.5 nm) monochromatic light source. The laser beam, polarized parallel to the fibers axis, was focused on the samples with the 50× objective onto a spot 1–2 μm in diameter. The acquired spectra were analyzed using the software of WIRE 3.3 to determine the peak areas.
XPS (ESCALAB 220i-XL, VG, UK) was performed to analyze the chemical composition of CF surface composition using monochromated Al Kα source (hν = 1486.6 eV) at a base pressure of 2 × 10−9 mbar. The pass energy was set at 180 eV for survey scan. High resolution spectra were obtained at a perpendicular take-off angle, using a pass energy of 20 eV and energy steps of 0.05 eV. The XPS peak version 4.1 software was used for data analysis.
Dynamic contact angle meter and tensiometer (DCAT21, Data Physics Instruments, Germany) were used to analyze the surface energy of CF. Deionized water (γd = 21.8 mN m−1, γ = 72.8 mN m−1) and diiodomethane (γd = 50.8 mN m−1, γ = 50.8 mN m−1, 99% purity, Alfa Aesar, USA) were used as test liquids. The surface energy (γf), its dispersion component (γdf) and polar component (γpf) of CF can be estimated from the measured dynamic contact angles of the test liquids and calculated by solving the following equation:
γl(1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) = 2(γplγpf)1/2 + 2(γdlγdf)1/2 θ) = 2(γplγpf)1/2 + 2(γdlγdf)1/2
| (1) |
| γf = γpf + γdf | (2) |
The tensile strength of CF is usually assessed by single fiber tensile tests. The samples were tested on a universal testing machine (5500R, Instron, USA) according to ASTM D3379-75. The experimental data generated by these tests has high scatter, mainly due to the presence of flaws along the fibers. Thus, the interpretation of the data must be done statistically. In this work, the results were analyzed by the two-parameter Weibull cumulative distribution function to fit the experimental results.
The interfacial shear strength (IFSS) was adopted to quantify the interfacial property between CF and matrix resin by the interfacial evaluation equipment (Tohei Sangyo Co. Ltd., Japan). Six individual fibers from each sample were fastened to a metal holder with adhesive tape to ensure they were kept straight. Epoxy resin (E-51) was then mixed with curing agent (H-256) in a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 (w/w) ratio to prepare microdroplets. The microdroplets were cured at 363 K for 2 h, 393 K for 2 h and 423 K for 3 h. The values of IFSS were calculated according to the following equation:
32 (w/w) ratio to prepare microdroplets. The microdroplets were cured at 363 K for 2 h, 393 K for 2 h and 423 K for 3 h. The values of IFSS were calculated according to the following equation:
The composite inter-laminar shear strength (ILSS) was measured on a universal testing machine (5569, Instron, USA) based on ASTM D2344. Specimen dimensions were 20 mm × 6 mm × 2 mm. The values of ILSS for the composites were calculated with the following equation:
Impact tests were carried out on a drop weight impact test system (9250HV, Instron, USA). The impact span is 40 mm. The drop weight was 3 kg and the velocity was 1 m s−1. The specimen dimensions were 55 mm × 6.5 mm × 2 mm. Each date was obtained from the average value of 5 specimens.
The surface morphologies and the fractured surface of the untreated and functionalized CFs were observed by SEM (Quanta 200FEG, Hitachi Instrument, Inc. Japan). The samples were coated with a thin conducted gold layer by sputtering prior to the SEM observation in order to capture a stable and clear image.
3. Results and discussion
3.1. Surface chemical elemental composition of CF
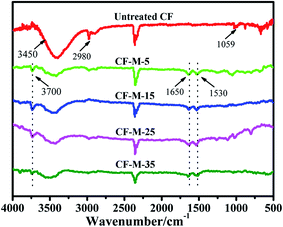
Surface functional groups analysis of CF was carried out by FTIR. The FTIR spectra can provide precise information of the material surface functional groups even at low concentrations.25 Fig. 2 shows the FTIR spectra of untreated and grafted CF. For untreated CF, some organic groups can be observed in addition to the bonds of carbon dioxide in the air. The bond around 3600–3100 cm−1 attributes to O–H stretching vibration of the absorbed water on fiber surface. The bonds range from 1059–1099 cm−1 and 2980–2800 cm−1 are corresponding to C–C and C–H stretching vibration, respectively. Compared with untreated CF, some new bonds can be observed for grafted CF. The bond centered at 3700 cm−1 corresponds to N–H stretching vibration of the amino groups in melamine. The bonds at 1650 and 1530 cm−1 are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and N–H bending vibration of the amide group, respectively.26 FTIR results confirm the reaction between CF-COCl and CF-NH2, and prove that melamine have been grafted onto the CF surface through covalent bonds.
O stretching vibration and N–H bending vibration of the amide group, respectively.26 FTIR results confirm the reaction between CF-COCl and CF-NH2, and prove that melamine have been grafted onto the CF surface through covalent bonds.
Raman spectroscopy is carried out to analyze the surface structure and the integrity of graphite structure. Fig. 3a shows the spectra of untreated fiber and grafted fiber. The spectra show two main peaks. The peak at 1330 cm−1 (D peak) is related to the defects and disordered carbonaceous structure. The other peak center at 1580 cm−1 (G peak) corresponds to the ordered graphitic structure.27 The intensity ratio of D and G peak (ID/IG, known as “R”) is attributed to the graphitization degree in the carbonaceous materials, the higher R value indicates the higher structurally disordered graphite crystallites in the CF.28 However, as shown in Fig. 3a, the overlap between D and G peak is serious, which is not conductive to analyze thoroughly, therefore, the spectra must be fitted. Fig. 3b–f show the fitted results. According to the spectra, D peak area of the grafted CF (Fig. 3c–f) is significantly increased when compared with that of untreated CF (Fig. 3b), which mainly due to the grafting of melamine. The grafting process induce amount of amino group onto the surface of CF, which could break the graphite structure of CF surface and lead to a higher disordered degree. In addition, a new peak around 1500–1550 cm−1 known as “A peak” can be observed, which is associated with amorphous carbonaceous structures, some hetero atoms on the CF surface or some original functional groups. The intensity ratio of A and G peak (IA/IG) is corresponding to the proportion of amorphous carbonaceous structures as well as some oxygen-containing and/or nitrogen containing functional groups on the CF surface.29 Table 1 shows the wavenumber of three peaks and the values of “ID/IG” and “IA/IG”. It can be seen that the R value of the grafted CF are much higher than that of untreated CF, which indicates the etching effect in the grafting process remove the carbonaceous structures on the surface of CF, and damage the ordered graphitic structure of the CF surface. Therefore, disordered carbonaceous components on CF surface are improved while the graphitic structure of CF is reduced. Meanwhile, the “IA/IG” values of CF also increase significantly after grafting melamine, which indicates amorphous carbonaceous structures (C–C and C–H) as well as some nitrogen containing functional groups in melamine have been grafted successfully onto CF surface. In addition, the value of “ID/IG” and “IA/IG” enhance gradually with the treated time increasing, demonstrating that the surface region structure of CF has changed and more melamine have been grafted onto CF in supercritical methanol.
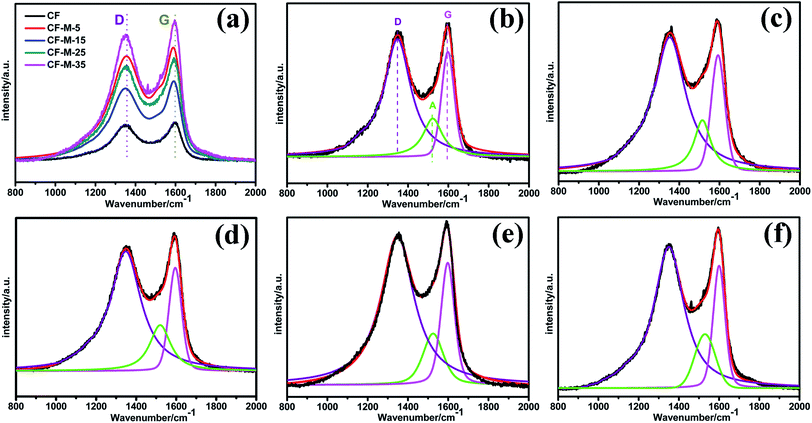 | ||
| Fig. 3 Raman spectra (a) and fitting spectra for all the samples (b) untreated CF, (c–f) CF-M-t (t = 5, 15, 25 and 35). | ||
| Samples | W (cm−1) | R = ID/IG | IA/IG | ||
|---|---|---|---|---|---|
| D | G | A | |||
| CF | 1348 | 1597 | 1522 | 3.32 | 0.64 |
| CF-M-5 | 1352 | 1593 | 1515 | 3.77 | 0.76 |
| CF-M-15 | 1350 | 1600 | 1530 | 3.81 | 0.81 |
| CF-M-25 | 1348 | 1595 | 1520 | 3.83 | 0.89 |
| CF-M-35 | 1348 | 1597 | 1525 | 3.87 | 0.97 |
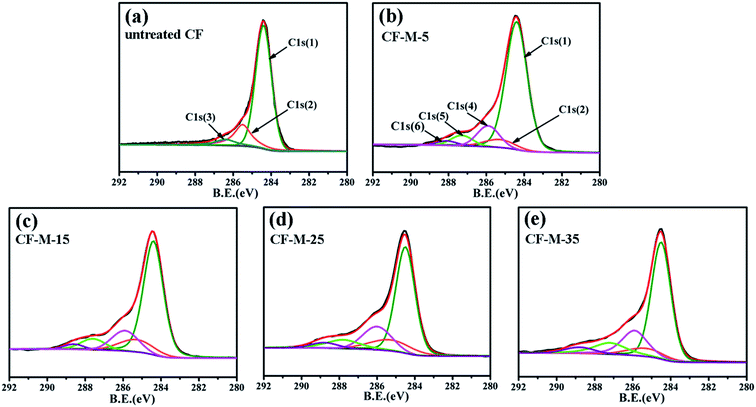
To further confirm that melamine have been grafted onto the CF surface through covalent bonds, untreated CF and grafted CF are investigated by XPS for determining the elemental/chemical surface compositions. The C1s XPS spectra of untreated CF and grafted CF are peak fitted to determine peak locations and areas in relation to specific binding energies for estimating the functional groups on fiber surface. The survey spectrum of C1s peak positions derived from peak deconvolution and fitting to data are list in Fig. 4. The content (at%) of relative groups is represented in Table 2. For untreated CF (Fig. 4a), aside from the main peak C1s (1) around at 284.4 eV which is assigned to Csp2 and Csp3 in the fiber structure, there are two another component peaks: C–C bonding of amorphous carbon around at 285.6 eV (peak C1s (2)) and C–O bond around at 286.3 eV (peak C1s (3)) which is attributed to atmospheric oxidation or residual oxides resulting from the CF purification process.30 The content of surface groups shown in Table 2 is 73.91%, 19.45%, 6.64%, respectively. After being grafted by melamine (Fig. 4b–e), there are deconvoluted three new mainly component peaks around at 285.8 eV (C1s (4)), 287.6 eV (C1s (5)) and 288.0 eV (C1s (6)) in the C1s core-level spectra. The peak around at 285.8 eV and 287.6 eV are attributed to C–N bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in melamine rings, respectively.31 The peak at 288.4 eV is assigned to amide group (NH–CO), indicating that chemical reaction happen between melamine and CF, which is consistent with the results of the FTIR discussed above.32 The results confirmed that amount of amino (–NH2) groups have been grafted on CF surface through grafting melamine in supercritical methanol, rather than coating. Moreover, compare the Fig. 4b–e, the content of NH–CO (peak C1s (6), 288.0 eV) show a trend of increasing, indicating more melamine have been reacted with the CF-COCl due to the increased treated time. The content of C–N, C
N bond in melamine rings, respectively.31 The peak at 288.4 eV is assigned to amide group (NH–CO), indicating that chemical reaction happen between melamine and CF, which is consistent with the results of the FTIR discussed above.32 The results confirmed that amount of amino (–NH2) groups have been grafted on CF surface through grafting melamine in supercritical methanol, rather than coating. Moreover, compare the Fig. 4b–e, the content of NH–CO (peak C1s (6), 288.0 eV) show a trend of increasing, indicating more melamine have been reacted with the CF-COCl due to the increased treated time. The content of C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and HN–CO bond are enhanced slightly when the CF are treated for 5 and 10 min according to Table 2. As treated for 25 min, the content shows an obvious increase and obtains the highest value (18.36%, 11.58% and 4.38%) at 35 min, suggesting more melamine have been grafted onto fiber surface with the increase of treated time and this is consistent with the XPS spectrums. These amino groups on fiber surface could greatly improve the wettability between fiber and matrix resin, and form strong chemical bonds at the interface through reacting with the epoxy groups in the matrix.
N and HN–CO bond are enhanced slightly when the CF are treated for 5 and 10 min according to Table 2. As treated for 25 min, the content shows an obvious increase and obtains the highest value (18.36%, 11.58% and 4.38%) at 35 min, suggesting more melamine have been grafted onto fiber surface with the increase of treated time and this is consistent with the XPS spectrums. These amino groups on fiber surface could greatly improve the wettability between fiber and matrix resin, and form strong chemical bonds at the interface through reacting with the epoxy groups in the matrix.
| Samples | Relative content of surface group (at%) | |||||
|---|---|---|---|---|---|---|
| C1s (1), C–C | C1s (2), C–C | C1s (3), C–O | C1s (4), C–N | C1s (5), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
C1s (6), NH–CO | |
| CF | 73.91 | 19.45 | 6.64 | — | — | — |
| CF-M-5 | 73.20 | 7.15 | — | 11.69 | 5.73 | 2.23 |
| CF-M-15 | 65.01 | 10.99 | — | 13.96 | 7.24 | 2.80 |
| CF-M-25 | 59.97 | 8.99 | — | 17.45 | 9.80 | 3.79 |
| CF-M-35 | 56.39 | 9.29 | — | 18.36 | 11.58 | 4.38 |
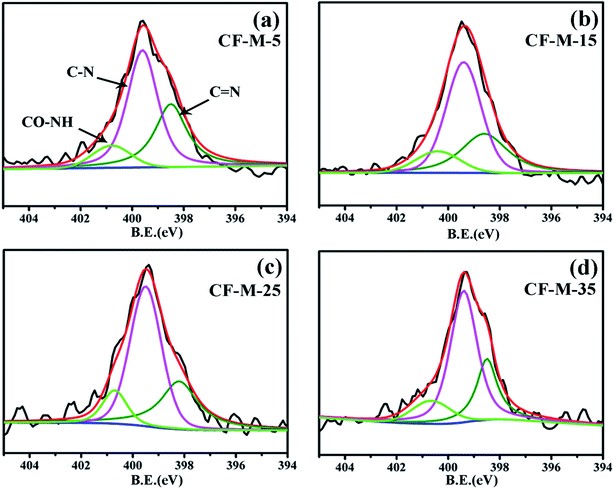
The chemical grafting of melamine was further confirmed by tracing N1s and the curve fitting for N1s peak are shown in Fig. 5. Besides the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (398.3 eV) and C–N bond (399.5 eV) in melamine rings, a peak at 400.6 eV can be also observed in Fig. 5a–d, which is assigned as amide group (NH–CO) due to the reaction of the amino and acyl chloride groups.33 The results are in good agreement with C1s peak deconvolution result.
N bond (398.3 eV) and C–N bond (399.5 eV) in melamine rings, a peak at 400.6 eV can be also observed in Fig. 5a–d, which is assigned as amide group (NH–CO) due to the reaction of the amino and acyl chloride groups.33 The results are in good agreement with C1s peak deconvolution result.
3.2. Surface topography of CF
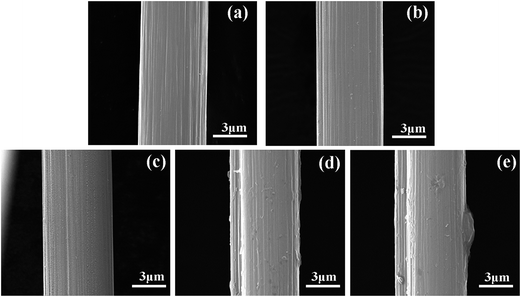
Surface topography's changes of the CF before and after modification are verified by SEM, and the results are shown in Fig. 6a–e, respectively. According to Fig. 6a, the untreated fiber exhibits a relatively neat and smooth surface, and a few narrow grooves parallel distribute along the longitudinal direction of the fiber. However, observation of the surface reveals some morphology alternations induced by grafting melamine. For CF-M-5, the CF surface (Fig. 6b) is not as smooth as that of untreated CF and a little melamine begin to be grafted onto the CF surface. Fig. 6c–e present the surface morphology of CF-M-15∼35. The surface roughness increases obviously and more melamine are grafted onto CF with the treated time increasing. In addition, it can be found that the grafted melamine distribute uniformly on CF at different directions except CF-M-35, on which a big block can be observed. The improved roughness can provide more contact points and enhance the mechanical interlocking between fiber and matrix resin, thus improve the interfacial adhesion of the resulting composites.3.3. Dynamic contact angle analysis (DCA) of CF
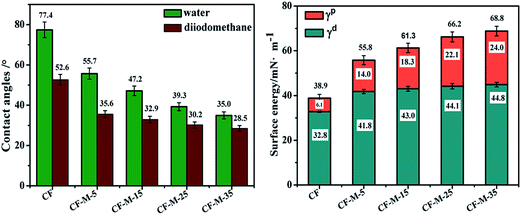
The fiber surface energy can be affected by surface functionality and roughness. The increase of the fiber surface energy can improve the wettability and interfacial adhesion between CF and matrix resin. Surface energy consists of two components: dispersive component (γd) and polar component (γp), which are determined by the contact angle based on the dynamic contact angle of double fluid method.34,35 Fig. 7 shows the contact angle (θ), surface energy (γ), dispersion component (γd) and polar component (γp) of untreated CF and grafted CF. As shown in Fig. 7, the contact angles with polar water and non-polar diiodomethane decrease from 77.4° to 35.0° and 52.6° to 28.5° after grafting melamine, respectively, indicating the wettability have been improved which mainly due to the improved polar of the CF and the increased surface roughness caused by grafting of melamine.The surface free energy of grafted CF are enhanced obviously in comparison with that of untreated CF. Moreover, the surface free energy increase with treated time increasing and the highest value is obtained at 35 min. This change of surface free energy is mainly caused by the increased polar component (γp). Based on the results discussed above, it can be found that melamine grafted CF have more polar amino groups and the amount of grafted amine groups increase with the time increasing, and this may be the main contributor for the improved polar component (γp). As amino groups are polar, the wettability between CF and matrix resin would be improved significantly. In addition, a tendency to increase in dispersion component (γd) can be observed from grafted CF according to the Fig. 7, indicating that melamine functionalization give rise to increased roughness in CF surface with the increasing of the treated time. The consequence is in accordance with SEM images. In summary, melamine functionalized give rise to increased fiber surface energy and improved wettability by massive amino groups, which could greatly enhance the interfacial adhension of composites.
3.4. Single fiber tensile testing of CF
Single fiber tensile tests are adopted to examine the effect of melamine grafting on the surface of CF. The fiber tensile testing results are presented in Table 3. The strength of a single CF obeys the single Weibull distribution.36 According to Table 3, CF-M-5 and CF-M-15 decrease slightly in fiber tensile strength compared with untreated CF. CF-M-5 has the lowest value of tensile strength (3.75 GPa), decrease by 1.83%. However, the result is much lower in comparison with that of the CF (5.79%) reported by K. M. Beggs et al.,37 suggesting the grafting process in supercritical methanol treatment unavoidably introduce some defects on the surface of fibers, which could decrease the fiber strength, but the reduction is very mild and can be neglected. As the treated time increase to 25 and 35 min, the tensile strength increase significantly, which can be contribute to more melamine are grafted on the surface of CF with the treated time increasing and they could protect the fibers effectively from introducing defects and damages on the surface structures of fiber. Single fiber tensile testing imply that melamine grafting in supercritical fluids would not lead to any discernible decrease and instead increase for the in-plane properties of the resulting composites at a certain treated time.| Samples | Ra | m | Σ0 | Expectation (GPa) |
|---|---|---|---|---|
| CF | 0.98 | −4.99 | 3.87 | 3.82 ± 0.19 |
| CF-M-5 | 0.97 | −4.97 | 3.85 | 3.75 ± 0.19 |
| CF-M-15 | 0.98 | −4.98 | 3.89 | 3.80 ± 0.19 |
| CF-M-25 | 0.99 | −5.06 | 3.87 | 3.88 ± 0.19 |
| CF-M-35 | 0.98 | −5.12 | 3.88 | 3.98 ± 0.2 |
3.5. Interfacial properties testing of composites
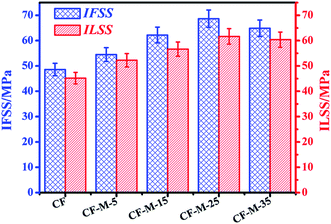
The interfacial shear strength (IFSS) of CF/epoxy composites have been tested in order to evaluate the influence of the melamine grafting on the interfacial adhesion properties between CF and matrix resin. The measured data of untreated CF and grafted CF are given in Fig. 8. After being grafted with melamine using supercritical method, the composite interfacial strength increase significantly. The IFSS increases from 48.6 to 54.5 MPa after treating for 5 min, enlarges at 12.1% in comparison with that of untreated CF, which could be attributed to the formation of considerable chemical bonding between the amine groups of melamine and epoxy matrix. After being treated with longer time, the composites show significant improvements in IFSS, by 28.0% and 41.3% for CF-M-15 and CF-M-25, respectively. The high interfacial mechanical properties enhancement can be mainly attributed to the chemical bonding. As the amount of grafted amine functional groups increase with the treated time, more amino groups can react with epoxy groups, which could improve the adhesion between CF and matrix resin. However, in case of CF-M-35, the IFSS of composite shows an decrease, which can be attributed to the “over coverage” of melamine onto CF.34 As referred in Fig. 6e, we can find melamine hasn't been grafted uniformly on fiber surface and some big blocks can be observed. The interfacial defect may take place within the blocks because of the van der Waals interaction among melamine molecules. Therefore, these blocks could have a negative effect on the interface properties of composite. Furthermore, the results also indicate that too long reaction time in supercritical fluids is inappropriate and this implies that supercritical methanol is an available medium to improve efficiency.The inter-laminar shear stress (ILSS) of the CF/epoxy composites have also been studied. As shown in Fig. 8, all the grafted fiber/epoxy resin composites show significantly increase in ILSS compared with that of untreated CF composites, and the increasing trend coincides with IFSS. CF-M-25 composite has the highest ILSS value (61.65 MPa), enhanced by 36.4% compared with that of untreated CF composite (45.2 MPa).
In addition, grafting melamine on the CF surface in supercritical methanol improve the interfacial properties apparently and efficiently. Meng et al. have reported that the IFSS and ILSS of CFs/epoxy composites were increased by 22.9% and 19.2%, respectively by treating the CF with triethylene tetramine in supercritical water/ethanol system.38 Servinis et al. have reported that the IFSS of CFs/epoxy composites was increased by 15.6% by grafting 2-fluoro-5-benzotrifluoride onto CF using in situ generated diazonium species.39 Zhao et al. have reported that the ILSS of CFs/epoxy composites was increased by 31.5% by grafting POSS on the CF surface.30 All of the percentage increase are lower than the observed IFSS and ILSS value here. The above testing results show that the modified method is potential and competitive to improve the interfacial properties of the CFs composite materials.
3.6. Impact property testing
The impact property tests are carried out to examine the effect of melamine grafting on impact resistance of composites. Fig. 9a shows the changes of impact strength for the composites reinforced by grafted CF. The untreated CF composite has the lowest impact strength, which mainly results from the weak interfacial adhesion discussed above. As a result, the interface of composites could crack easily under a small load. For grafted CF composites, the impact strength increase along with the reaction time. CF-M-35 composite has the highest impact strength (74.6 kJ m−2), increase by 23.7% compared with that of untreated CF composite due to the surface treatment with melamine. Furthermore, the impact strength of CF-M-35 composite show an increase of 15.56% in comparison with that of the CF composite (64.56 kJ m−2) which grafted HMTA on the surface of CF by Ma et al.29 These results indicate that the melamine interface could induce some smaller cracks to prevent the crack tips from contacting the fiber surface directly and make the crack path deviate away from the CF surface to the interface region. As the amount of melamine on fiber interface increases, the fracture energy could be absorbed more efficiently as a result of more cracks are induced. Therefore, the composites impact strength is increased.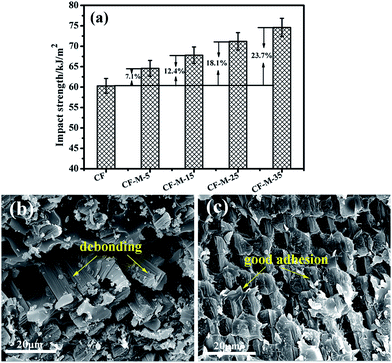 | ||
| Fig. 9 Impact test results of composites (a) and SEM micrographs of fracture surfaces (b) untreated CF, (c) CF-M-35. | ||
The impact fracture surface of composites have also been investigated by SEM to further confirm the enhancement. Fig. 9b and c shows the fractured surface of untreated CF and CF-M-35 composites which have the lowest and the highest value of impact strength. For untreated CF composites (Fig. 9b), the fibers are pulled out from the matrix in some area and remain some big holes. Furthermore, the interface de-bonding between fibers and matrix is obvious and the de-bonding CF surface are almost clean, suggesting a weak interfacial adhesion. Comparatively, the interface of CF-M-35 composite (Fig. 9c) improves significantly. The flat fracture surface can be observed and the fiber and matrix remains closely integrated together, indicating that melamine grafting can improve the adhesion between the grafted CF and matrix resin.
4. Conclusions
In this study, the surface modification of CF by grafting melamine in supercritical methanol was investigated. FT-IR, Raman, XPS and SEM study of CF surface confirmed that melamine was grafted successfully on the CF surface and improved the surface roughness. The surface energy enhanced with the increase of treated time, which suggested the functional groups of the CF surface increased obviously after modification. IFSS, ILSS and impact strength of grafted CF/epoxy composites were enhanced about 41.3%, 36.4% and 23.7%, respectively. The reinforcing and toughening mechanisms were also explored. In addition, the supercritical treatment did not lead to any discernible decrease in tensile strength. The strong interfacial interaction between grafted CF and matrix resin greatly improved the interfacial properties of CF reinforced composites. This processing of melamine grafted CF in supercritical methanol will provide a novel and effective approach to functionalize the CF and improve the interfacial performance of the composites.Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 21174034).References
- X. Q. Zhang, H. B. Xu and X. Y. Fan, RSC Adv., 2014, 4, 12198–12205 RSC.
- K. H. Wong, S. D. Mohammed, S. J. Pickering and R. Brooks, Compos. Sci. Technol., 2012, 72, 835–844 CrossRef CAS.
- Z. Liu, C. Tang, P. Chen, Q. Yu and W. K. Li, RSC Adv., 2014, 4, 26881–26887 RSC.
- W. Z. Qin, F. Vautard, P. Askeland, J. R. Yu and L. Drzal, RSC Adv., 2015, 5, 2457–2465 RSC.
- L. C. Ma, L. H. Meng, D. P. Fan, J. M. He, J. L. Yu, M. W. Qi and Y. D. Huang, Appl. Surf. Sci., 2014, 296, 61–68 CrossRef CAS.
- Y. W. Wang, L. H. Meng, L. Q. Fan, L. C. Ma, M. W. Qi, J. L. Yu and Y. D. Huang, Appl. Surf. Sci., 2014, 316, 366–372 CrossRef CAS.
- H. B. Lu, W. L. Yin, W. M. Huang and J. S. Leng, RSC Adv., 2013, 3, 21484–21488 RSC.
- G. S. Wu, L. C. Ma, L. Liu, Y. W. Wang, F. Xie, Z. X. Zhong and Y. D. Huang, Composites, Part B, 2015, 82, 50–58 CrossRef CAS.
- S. Osbeck, R. H. Bradley, C. Liu, H. Idriss and S. Ward, Carbon, 2011, 49, 4322–4330 CrossRef CAS.
- Z. W. Xu, Y. D. Huang, C. H. Zhang, L. Liu, Y. H. Zhang and L. Wang, Compos. Sci. Technol., 2007, 67, 3261–3270 CrossRef CAS.
- W. Wu, H. L. Jin, J. G. Zhang and Z. T. Yu, Polym.-Plast. Technol. Eng., 2012, 51, 556–559 CrossRef CAS.
- G. J. Ehlert, U. Galan and H. A. Sodano, ACS Appl. Mater. Interfaces, 2013, 5, 635–645 CAS.
- B. X. Yang, J. H. Shi and S. H. Goh, Compos. Sci. Technol., 2008, 68, 2490–2497 CrossRef CAS.
- B. Roy, P. Bairi and A. K. Nandi, RSC Adv., 2014, 4, 1708–1734 RSC.
- F. Adam, K. M. Hello and H. Osman, Appl. Catal., 2010, 382, 115–121 CrossRef CAS.
- A. Grondein and D. Bélanger, Carbon, 2012, 50, 4335–4342 CrossRef CAS.
- C. Aymonier, A. S. Loppinet, H. Revern, Y. Garrabos and F. Cansell, J. Supercrit. Fluids, 2006, 38, 242–251 CrossRef CAS.
- G. Madras, C. Kolluru and R. Kumar, Fuel, 2004, 83, 2029–2033 CrossRef CAS.
- T. Goto and T. Yamazaki, J. Soc. Rubber Ind., Jpn., 2004, 77, 359–364 CrossRef CAS.
- G. Brunner, J. Supercrit. Fluids, 2009, 47, 382–390 CrossRef CAS.
- L. H. Meng, D. P. Fan and Y. D. Huang, Appl. Surf. Sci., 2012, 261, 415–421 CrossRef CAS.
- J. R. Hyde, E. Lester, S. Kingman, S. Pickering and K. H. Wong, Composites, Part A, 2006, 37, 2171–2175 CrossRef.
- Y. Y. Liu, G. H. Shan and L. H. Meng, Mater. Sci. Eng., 2009, 520, 179–183 CrossRef.
- J. L. Yu, L. H. Meng and Y. D. Huang, Composites, Part B, 2014, 60, 261–267 CrossRef CAS.
- S. L. Gao, E. Mäder and S. F. Zhandarov, Carbon, 2004, 42, 515–529 CrossRef CAS.
- M. C. Paiva, C. A. Bernardo and M. Nardin, Carbon, 2000, 38, 1323–1337 CrossRef CAS.
- L. M. Malard, M. A. Pimenta, G. Dresselhaus and M. S. Dresselhaus, Phys. Rep., 2009, 473, 51–87 CrossRef CAS.
- J. E. Liu, Y. L. Tian, Y. J. Chen, J. Y. Liang, L. F. Zhang and H. Fong, Mater. Chem. Phys., 2010, 122, 548–555 CrossRef CAS.
- L. C. Ma, L. H. Meng, Y. W. Wang, G. S. Wu, D. P. Fang, Y. J. Li, M. W. Qi and Y. D. Huang, RSC Adv., 2014, 4, 39156–39166 RSC.
- F. Zhao and Y. D. Huang, J. Mater. Chem., 2011, 21, 3695–3703 RSC.
- A. Deryło-Marczewska, J. Goworek, S. Pikus and E. Kobylas, Langmuir, 2002, 18, 7538–7543 CrossRef.
- Q. Y. Peng, X. D. He, Y. B. Li, C. Wang, R. G. Wang and P. A. Hu, J. Mater. Chem., 2012, 22, 5928–5931 RSC.
- D. M. Feng, Z. M. Zhou and M. P. Bo, Polym. Degrad. Stab., 1995, 50, 65–70 CrossRef CAS.
- A. Kafi, M. Huson, C. Creighton, J. Khoo, L. Mazzola, T. Gengenbach, F. Jones and B. Fox, Compos. Sci. Technol., 2014, 94, 89–95 CrossRef CAS.
- Q. Y. Peng, Y. B. Li, X. D. He, H. Z. Lv, P. A. Hu, Y. Y. Shang, C. Wang, R. G. Wang, T. Sritharan and S. Y. Du, Compos. Sci. Technol., 2013, 74, 37–42 CrossRef CAS.
- D. W. Jiang, Y. D. Huang, S. Y. Wei and Z. H. Guo, J. Mater. Chem., 2014, 2, 18293–18303 RSC.
- K. M. Beggs, L. Servinis, T. R. Gengenbach, M. G. Huson, B. L. Fox and L. C. Henderson, Compos. Sci. Technol., 2015, 118, 31–38 CrossRef CAS.
- L. H. Meng, D. P. Fan, C. H. Zhang, Z. X. Jiang and Y. D. Huang, Composites, Part B, 2014, 56, 575–581 CrossRef CAS.
- L. Servinis, L. C. Henderson, L. M. Andrighetto, M. G. Huson, T. R. Gengenbach and B. L. Fox, J. Mater. Chem. A, 2015, 3, 3360–3371 CAS.
| This journal is © The Royal Society of Chemistry 2016 |