Graphene-constructed flower-like porous Co(OH)2 with tunable hierarchical morphologies for supercapacitors†
Qi Huang,
Jinzuan Wang,
Fan Liu,
Xing Chang,
Hongyan Chen,
Hualin Lin* and
Sheng Han*
School of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, China. E-mail: lhl6534@163.com; hansheng654321@sina.com
First published on 3rd February 2016
Abstract
Graphene-constructed flower-like porous Co(OH)2 (GFC) composites were prepared using a one-pot hydrothermal process. The flower-like porous Co(OH)2 with a hierarchical structure was perfectly constructed using graphene sheets. This strategy facilitated the large-scale synthesis of GFC composites. The morphologies of Co(OH)2 ranged from flower petals to large flower-like microspheres. The morphologies were easily controlled by changing the weight ratio of graphene oxide to cobalt salt. The flowers were approximately 5–10 μm in diameter and composed of numerous thin nanoplates. The GFC, as an electrode material for supercapacitors, showed a high specific capacitance of 480 F g−1 at 1 A g−1 and outstanding cycling performance with 93.5% capacitance retained over 1000 cycles. The outstanding electrochemical performance of GFC could be attributed to the large specific surface area of the GFC and synergistic interaction between uniformly dispersed Co(OH)2 and graphene.
1. Introduction
Supercapacitors have attracted much attention as indispensable energy storage devices because of their high power densities, fast charging/discharging time, and long cycle life.1–3 Various potential candidates for supercapacitor electrodes, such as carbon materials, conducting polymers, and transition metal oxides/hydroxides, have been explored. Transition metal oxides/hydroxides, such as RuO2,4 Fe2O3,5,6 Co3O4,7,8 TiO2,9,10 Co(OH)2,11 and Ni(OH)2,12 can be extensively used for energy storage as typical active materials for supercapacitor electrodes. Among these materials, Co(OH)2 is considered a promising electroactive material given its relatively low cost, well-defined electrochemical redox activity, layered structure with large interlayer spacing, and high theoretical capacitance (3460 F g−1).13–15 Structure, shape, and size significantly affect properties of materials and their applications. Numerous Co(OH)2 micro- and nanostructures with tunable morphologies, such as nanosheets,16 flower-like microspheres,17 nanoflakes,18 nanoparticles,19 and nanofilms,20,21 have been synthesized through various routes. However, the application of Co(OH)2 as a supercapacitor electrode is limited because of the low conductivity and strong agglomeration during charge and discharge processes.Combining Co(OH)2 with conductive substrates can efficiently enhance the conductivity of electrodes and remarkably influence its electrochemical performance. Graphene is a monolayer of carbon atoms arranged in a hexagonal lattice. This compound has been widely employed as a supporting substrate because of its unique electrical and mechanical properties, large specific surface area (theoretical value of 2630 m2 g−1), and high chemical stability.22–24 Several Co(OH)2/graphene hybrids or composites, such as Co(OH)2 nanoparticle/graphene,25 Co(OH)2 nanosheet/graphene,26 Co(OH)2 nanorod/graphene,27 Co(OH)2 nanoflake/graphene,14 and Co(OH)2 hexagonal platelet/graphene,28,29 have been intensively investigated as electrode materials for supercapacitors or lithium-ion batteries. These studies showed a considerable enhancement of supercapacitor or battery capacities. Thus, the design and synthesis of Co(OH)2 with special morphology anchored on graphene are desirable to improve electron transport and contribute to the energy storage of the whole composite to achieve high-performance supercapacitors.
In this paper, we report the large-scale synthesis, high specific surface area, and excellent electrochemical performance of graphene-constructed flower-like porous Co(OH)2 (GFC) composites. The composites were prepared using graphene oxide (GO) as a precursor for reduced graphene oxide (RGO) and cobalt acetate as a single source precursor of Co(OH)2 under hydrothermal conditions. The flower-like Co(OH)2 (FC) with hierarchical structure and size of approximately 5–10 μm were uniformly distributed on the surface of graphene nanosheets (GNs). We also performed control experiments by varying the weight ratios of GO to cobalt salt to explore the growth morphologies of the hierarchical structures. The structures and morphologies of the GFC composites were examined by field emission scanning electron microscopy, transmission electron microscopy (TEM), and X-ray powder diffraction (XRD). Finally, the application of such Co(OH)2 with different hierarchical morphologies distributed on the GNs for supercapacitors was also investigated.
2. Experimental
2.1. Materials
Graphite flakes were bought from Sigma Aldrich (USA). Co(Ac)2·4H2O, ammonia solution (NH3·H2O, 25–28%), KMnO4, NaNO3, 37% HCl, 98% H2SO4, 30% H2O2 were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All the chemical regents were of analytical grade and were used as received without further purification. All aqueous solutions were prepared with deionized (DI) water.2.2. Synthesis of GFC composites
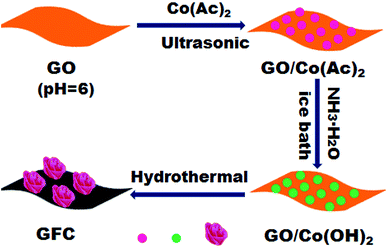
GO was prepared from natural graphite flakes using a modified Hummers method. The synthesis method was illustrated in Scheme 1. Firstly, 360 mg Co(Ac)2·4H2O ware dissolved in 30 mL DI water, and then mixed together with 40 mL of aqueous GO dispersion (1.50 mg mL−1, pH = 6), followed by ultrasonication for 30 min to form a homogeneous suspension. Subsequently, 5 mL ammonia solution was added slowly, the mixture was stirred for 30 min in an ice bath. Then, the 75 mL mixture was transferred into 100 mL Teflon lined stainless steel autoclaves for hydrothermal treatment at 180 °C for 12 h, and then cooled to room temperature naturally. Next, the resulting suspension was centrifuged and washed with DI water for several times and dried at 80 °C to afford GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). For comparison, the GFC (1
6). For comparison, the GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), GFC (1
5), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and GFC (1
10), and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) were synthesized by the same procedure with different weight ratio of GO to Co(Ac)2·4H2O. Pure flower-like porous Co(OH)2 (FC) was also prepared by the same chemical process only without GO suspensions. The reduced graphene oxide (RGO) could be prepared by hydrothermal treatment of GO.
20) were synthesized by the same procedure with different weight ratio of GO to Co(Ac)2·4H2O. Pure flower-like porous Co(OH)2 (FC) was also prepared by the same chemical process only without GO suspensions. The reduced graphene oxide (RGO) could be prepared by hydrothermal treatment of GO.
2.3. Characterization
The morphology was investigated with a field-emission scanning electron microscopy (FE-SEM, FEI, Sirion 200) and transmission electron microscopy (TEM, JEOL-2100). X-ray diffraction (XRD) analysis was performed on a Rigaku X-ray diffractometer with Cu-Kα irradiation (λ = 0.15406 nm) at 40 kV, 20 mA over the 2θ range from 5 to 80°. Thermogravimetric analysis (TGA) was characterized a TA Q5000IR with a heating rate of 10 °C min−1 under flowing air. The X-ray photoelectron spectrum (XPS) was taken with an X-ray photoelectron spectrometer (AXIS ULTRA DLD, Kratos). And nitrogen adsorption/desorption isotherms at 77 K by using a Micrometrics ASAP 2010.2.4. Electrochemical measurements
The working electrodes were prepared by mixing the composites, carbon black, and polytetrafluoroethylene (PTFE) at a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dissolved in the ethanol to form a slurry and pressing the slurry onto a Ni foam (1.0 cm × 1.0 cm), and then drying under vacuum at 60 °C for 12 h. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were measured by a CHI 660D electrochemical workstation (Shanghai Chenhua Co. Ltd., China). Galvanostatic charge/discharge measurement was performed on a LAND 2001A system (Wuhan, China) in a voltage rang of 0–0.4 V. The electrolyte was 6 M KOH. The mass of electroactive GFC in each electrode was about 2.0 mg. The reference electrode and counter electrode were a saturated calomel electrode (SCE) and a platinum plate, respectively.
1 dissolved in the ethanol to form a slurry and pressing the slurry onto a Ni foam (1.0 cm × 1.0 cm), and then drying under vacuum at 60 °C for 12 h. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were measured by a CHI 660D electrochemical workstation (Shanghai Chenhua Co. Ltd., China). Galvanostatic charge/discharge measurement was performed on a LAND 2001A system (Wuhan, China) in a voltage rang of 0–0.4 V. The electrolyte was 6 M KOH. The mass of electroactive GFC in each electrode was about 2.0 mg. The reference electrode and counter electrode were a saturated calomel electrode (SCE) and a platinum plate, respectively.
3. Results and discussion
3.1. Structural properties
The XRD patterns of GO, RGO, FC, and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) composite are shown in Fig. 1. The intense peak of GO at 2θ = 11° corresponded to the (001) reflection. The (001) peak at 11° disappeared, and a broad (002) peak appeared at 23.6°, confirming the conversion of GO to RGO. The peaks of FC at 2θ = 19.05°, 32.47°, 37.92°, 51.36°, 57.91°, 59.57°, 61.53°, 69.51°, and 71.79° corresponded to the (001), (100), (101), (102), (110), (003), (111), (103), and (112) planes, which were well indexed to the hexagonal phase of β-Co(OH)2 (JCPDS card no. 30-0443). All peaks of β-Co(OH)2 in the GFC (1
6) composite are shown in Fig. 1. The intense peak of GO at 2θ = 11° corresponded to the (001) reflection. The (001) peak at 11° disappeared, and a broad (002) peak appeared at 23.6°, confirming the conversion of GO to RGO. The peaks of FC at 2θ = 19.05°, 32.47°, 37.92°, 51.36°, 57.91°, 59.57°, 61.53°, 69.51°, and 71.79° corresponded to the (001), (100), (101), (102), (110), (003), (111), (103), and (112) planes, which were well indexed to the hexagonal phase of β-Co(OH)2 (JCPDS card no. 30-0443). All peaks of β-Co(OH)2 in the GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) composite were observed with an additional crystallinity induced at 2θ of 23.6°, corresponding to the (002) plane of RGO. The other two peaks at 36.78° and 65.26° could be attributed to some impure phases corresponding to CoOOH formation.28
6) composite were observed with an additional crystallinity induced at 2θ of 23.6°, corresponding to the (002) plane of RGO. The other two peaks at 36.78° and 65.26° could be attributed to some impure phases corresponding to CoOOH formation.28
The TG curves of FC and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) within the temperature range of 50–700 °C are shown in Fig. 2. FC showed a two-step weight loss caused by dehydration and decomposition of Co(OH)2. The weight loss at 50–150 °C was related to the adsorbed water of Co(OH)2, whereas the weight loss of 13.4% from 150 °C to 500 °C corresponded to the loss of the –OH group of Co(OH)2 and was accompanied with the conversion to Co3O4.30 Moreover, the strong weight loss of GFC (1
6) within the temperature range of 50–700 °C are shown in Fig. 2. FC showed a two-step weight loss caused by dehydration and decomposition of Co(OH)2. The weight loss at 50–150 °C was related to the adsorbed water of Co(OH)2, whereas the weight loss of 13.4% from 150 °C to 500 °C corresponded to the loss of the –OH group of Co(OH)2 and was accompanied with the conversion to Co3O4.30 Moreover, the strong weight loss of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) of approximately 33.7% from 150 °C to 500 °C was assigned to the oxidation of Co(OH)2 and combustion of graphene. Thus, the graphene content in GFC (1
6) of approximately 33.7% from 150 °C to 500 °C was assigned to the oxidation of Co(OH)2 and combustion of graphene. Thus, the graphene content in GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) was evaluated to be approximately 21.7 wt%.
6) was evaluated to be approximately 21.7 wt%.
The morphology and structures of FC and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) were observed by SEM and high-resolution TEM (HRTEM) images (Fig. 3). The SEM images of FC are shown in Fig. 3a and b. The FC microspheres were irregular in shape and constituted nanoplates that were not completely separated from each other but somehow agglomerated. The SEM image of the GFC (1
6) were observed by SEM and high-resolution TEM (HRTEM) images (Fig. 3). The SEM images of FC are shown in Fig. 3a and b. The FC microspheres were irregular in shape and constituted nanoplates that were not completely separated from each other but somehow agglomerated. The SEM image of the GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) product revealed that FCs with several hierarchical structures of 5–10 μm in diameter were uniformly distributed on the surface of GNs (Fig. 3c). Simultaneously, the flowers of Co(OH)2 in the GFC (1
6) product revealed that FCs with several hierarchical structures of 5–10 μm in diameter were uniformly distributed on the surface of GNs (Fig. 3c). Simultaneously, the flowers of Co(OH)2 in the GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) were encapsulated in the wrinkles of GNs. The encapsulated graphene sheet could efficiently prevent aggregation of flowers and prevent direct contact between FC and the electrolyte. The SEM image at higher magnification shows that the flower-like structure was composed of two-dimensional petaloid nanoplates (Fig. 3d). The flower-like morphology might be the result of nano/microsheets agglomerating together to form the interesting structure. The TEM image in Fig. 3e shows that the Co(OH)2 nanoplate of GFC (1
6) were encapsulated in the wrinkles of GNs. The encapsulated graphene sheet could efficiently prevent aggregation of flowers and prevent direct contact between FC and the electrolyte. The SEM image at higher magnification shows that the flower-like structure was composed of two-dimensional petaloid nanoplates (Fig. 3d). The flower-like morphology might be the result of nano/microsheets agglomerating together to form the interesting structure. The TEM image in Fig. 3e shows that the Co(OH)2 nanoplate of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) was effectively wrapped by GNs, indicating the strong adhesion between FCs and GNs. The HRTEM image of GFC (1
6) was effectively wrapped by GNs, indicating the strong adhesion between FCs and GNs. The HRTEM image of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) was taken to examine the crystal structure of Co(OH)2 nanoplates (Fig. 3f). The typical lattice fringe spacing was measured as approximately 0.46 nm, which was consistent with the (001) crystalline planes.31 The strongest peak in the XRD pattern was also (001). The crystal planes were not continuous in the whole crystal, and some pores formed in the nanoplate. Thus, the Co(OH)2 hexagonal plate grew mainly along the (001) direction.32
6) was taken to examine the crystal structure of Co(OH)2 nanoplates (Fig. 3f). The typical lattice fringe spacing was measured as approximately 0.46 nm, which was consistent with the (001) crystalline planes.31 The strongest peak in the XRD pattern was also (001). The crystal planes were not continuous in the whole crystal, and some pores formed in the nanoplate. Thus, the Co(OH)2 hexagonal plate grew mainly along the (001) direction.32
The physical properties and composition of the GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) composite were further analyzed by XPS. This technique can provide the chemical composition, chemical state, and binding energy of the material. The XPS spectrum of GFC (1
6) composite were further analyzed by XPS. This technique can provide the chemical composition, chemical state, and binding energy of the material. The XPS spectrum of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) is shown in Fig. 4, which indicated that the composite contained C, O, and Co as the main components. The XPS spectra of Co 2p (inset of Fig. 4) revealed that the peaks of Co 2p3/2 and Co 2p1/2 were centered at 781.6 and 797.1 eV (with a splitting of 15.5 eV), respectively, which were ascribed to the presence of Co2+ chemical state, indicating Co(OH)2 formation.33 The results further validated the existence of Co(OH)2 in the composite.
6) is shown in Fig. 4, which indicated that the composite contained C, O, and Co as the main components. The XPS spectra of Co 2p (inset of Fig. 4) revealed that the peaks of Co 2p3/2 and Co 2p1/2 were centered at 781.6 and 797.1 eV (with a splitting of 15.5 eV), respectively, which were ascribed to the presence of Co2+ chemical state, indicating Co(OH)2 formation.33 The results further validated the existence of Co(OH)2 in the composite.
The microstructures of FC and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) were further investigated by N2 adsorption–desorption analysis (Fig. 5). The isotherm of FC and GFC (1
6) were further investigated by N2 adsorption–desorption analysis (Fig. 5). The isotherm of FC and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) showed a type IV characteristic with a H3 hysteresis loop, proving that the products were BET and BJH methods. The specific surface areas of FC and GFC (1
6) showed a type IV characteristic with a H3 hysteresis loop, proving that the products were BET and BJH methods. The specific surface areas of FC and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) were approximately 39.1 and 97.9 m2 g−1, respectively, indicating that the added graphene and FC with several hierarchical structures played a very important role in improving the specific surface area. The pore size distribution plot of FC and GFC (1
6) were approximately 39.1 and 97.9 m2 g−1, respectively, indicating that the added graphene and FC with several hierarchical structures played a very important role in improving the specific surface area. The pore size distribution plot of FC and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) also verified the presence of micro- and mesopores in the material with pore size distribution maxima centered at ∼4 nm. The results showed that Co(OH)2 nanoplates should also be porous. The advantage of such decorated Co(OH)2 is that the Co(OH)2 flowers could be wrapped by graphene with a large area. This process then led to the formation of paths for ion transport between the electrolyte and active material. The morphologies and structures of GFC (1
6) also verified the presence of micro- and mesopores in the material with pore size distribution maxima centered at ∼4 nm. The results showed that Co(OH)2 nanoplates should also be porous. The advantage of such decorated Co(OH)2 is that the Co(OH)2 flowers could be wrapped by graphene with a large area. This process then led to the formation of paths for ion transport between the electrolyte and active material. The morphologies and structures of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), GFC (1
5), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and GFC (1
10), and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) were also observed by SEM to explore the growth morphologies of the hierarchical structures (Fig. 6). Numerous interlaced petaloid plates of Co(OH)2 of GFC (1
20) were also observed by SEM to explore the growth morphologies of the hierarchical structures (Fig. 6). Numerous interlaced petaloid plates of Co(OH)2 of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were dispersed on the surface of GNs (Fig. 6a and b). The rose porous FC microspheres with a ringent hierarchical structure in the GFC (1
5) were dispersed on the surface of GNs (Fig. 6a and b). The rose porous FC microspheres with a ringent hierarchical structure in the GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) composite and almost all flowers were perfectly distributed on the surface of GNs (Fig. 6c and d). Moreover, the FCs in the GFC (1
10) composite and almost all flowers were perfectly distributed on the surface of GNs (Fig. 6c and d). Moreover, the FCs in the GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were obviously encapsulated in the wrinkles of graphene sheets, further confirming the strong adhesion between FCs and GNs (Fig. S1†). Co(OH)2 of GFC (1
10) were obviously encapsulated in the wrinkles of graphene sheets, further confirming the strong adhesion between FCs and GNs (Fig. S1†). Co(OH)2 of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) displayed a large flower-like and dense closed microsphere structure with a diameter of approximately 10 μm when the mass of cobalt salt was increased further (Fig. 6e and f). In the current study, the FC with tunable hierarchical morphologies that ranged from flower petals to large flower-like microspheres could be constructed using graphene. Otherwise, the morphology of pure Co(OH)2 presented irregular flower-like microsphere structures. The hierarchical nanosheets of FC became denser when the mass of cobalt salt was gradually increased (Fig. S2†).
20) displayed a large flower-like and dense closed microsphere structure with a diameter of approximately 10 μm when the mass of cobalt salt was increased further (Fig. 6e and f). In the current study, the FC with tunable hierarchical morphologies that ranged from flower petals to large flower-like microspheres could be constructed using graphene. Otherwise, the morphology of pure Co(OH)2 presented irregular flower-like microsphere structures. The hierarchical nanosheets of FC became denser when the mass of cobalt salt was gradually increased (Fig. S2†).
 | ||
Fig. 6 Low and high magnification SEM images of samples, (a and b) GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); (c and d) GFC (1 5); (c and d) GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10); and (e and f) GFC (1 10); and (e and f) GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), respectively. 20), respectively. | ||
3.2. Electrochemical properties
Cyclic voltammetry (CV), galvanostatic charge/discharge (GCD), and electrochemical impedance spectroscopy (EIS) were adopted to evaluate the electrochemical performance of the prepared composites as active materials for supercapacitor electrodes in a three-electrode system. Fig. 7a presents the CV curves of FC, GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), GFC (1
5), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), GFC (1
6), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and GFC (1
10), and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) electrodes with voltage ranging from −0.2 V to 0.5 V at a scan rate of 10 mV s−1 in 6.0 M KOH electrolyte. The CV curves of all electrode active materials were observed with forward and reverse sweeps, in which two prominent redox peaks indicated the redox couple of Co(OH)2/CoO2 according to the following equations:34
20) electrodes with voltage ranging from −0.2 V to 0.5 V at a scan rate of 10 mV s−1 in 6.0 M KOH electrolyte. The CV curves of all electrode active materials were observed with forward and reverse sweeps, in which two prominent redox peaks indicated the redox couple of Co(OH)2/CoO2 according to the following equations:34| Co(OH)2 + OH− ↔ CoOOH + H2O + e− |
| CoOOH + OH− ↔ CoO2 + H2O + e− |
The GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) electrode exhibited the largest integral area and the highest capacitance in the closed CV loops, which were associated with the morphology, structure, content, and specific surface area of GFC (1
6) electrode exhibited the largest integral area and the highest capacitance in the closed CV loops, which were associated with the morphology, structure, content, and specific surface area of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) at various scan rates (Fig. 7b). The oxidation and reduction peaks shifted positively and negatively with the increase in the scan rate from 5 mV s−1 to 100 mV s−1. This trend indicated that fast redox reactions occurred at the electrolyte/electrode interface. Furthermore, the redox peaks of the CV curves did not change distinctly, showing the excellent rate performance of the electrode.
6) at various scan rates (Fig. 7b). The oxidation and reduction peaks shifted positively and negatively with the increase in the scan rate from 5 mV s−1 to 100 mV s−1. This trend indicated that fast redox reactions occurred at the electrolyte/electrode interface. Furthermore, the redox peaks of the CV curves did not change distinctly, showing the excellent rate performance of the electrode.
The GCD plots of FC, GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), GFC (1
5), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), GFC (1
6), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and GFC (1
10), and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) electrodes at a current density of 1 A g−1 are shown in Fig. 7c. The longest discharge time of GFC (1
20) electrodes at a current density of 1 A g−1 are shown in Fig. 7c. The longest discharge time of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) indicated that it had the highest electrochemical performance, which was consistent with the CV results. The charge–discharge coulombic efficiency of FC, GFC (1
6) indicated that it had the highest electrochemical performance, which was consistent with the CV results. The charge–discharge coulombic efficiency of FC, GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), GFC (1
5), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), GFC (1
6), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and GFC (1
10), and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) at 1 A g−1 were calculated to be approximately 82.4%, 66.8%, 61.6%, 75.5%, and 73.3%, respectively. The specific capacitance can be calculated as C = IΔt/mΔV, where I is the charge/discharge current, Δt is the time of discharge, m is the mass of active material in the working electrode, and ΔV is the potential window. The calculated specific capacitances of FC, GFC (1
20) at 1 A g−1 were calculated to be approximately 82.4%, 66.8%, 61.6%, 75.5%, and 73.3%, respectively. The specific capacitance can be calculated as C = IΔt/mΔV, where I is the charge/discharge current, Δt is the time of discharge, m is the mass of active material in the working electrode, and ΔV is the potential window. The calculated specific capacitances of FC, GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), GFC (1
5), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), GFC (1
6), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and GFC (1
10), and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) at different current densities from 1 A g−1 to 20 A g−1 are plotted in Fig. 7e. The specific capacitance for all the electrodes decreased with increasing charge/discharge current density. The specific capacitances for FC, GFC (1
20) at different current densities from 1 A g−1 to 20 A g−1 are plotted in Fig. 7e. The specific capacitance for all the electrodes decreased with increasing charge/discharge current density. The specific capacitances for FC, GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), GFC (1
5), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), GFC (1
6), GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and GFC (1
10), and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) at 1 A g−1 were 94, 378, 480, 273, and 132 F g−1, respectively. The GFC (1
20) at 1 A g−1 were 94, 378, 480, 273, and 132 F g−1, respectively. The GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) electrode demonstrated the highest specific capacitance. The charge/discharge behavior of GFC (1
6) electrode demonstrated the highest specific capacitance. The charge/discharge behavior of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) at different current densities is depicted in Fig. 7d. The specific capacitances of GFC (1
6) at different current densities is depicted in Fig. 7d. The specific capacitances of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) were calculated to be approximately 480, 437, 418, 365, and 300 F g−1 at current densities of 1, 2, 5, 10, and 20 A g−1, respectively. Moreover, the charge–discharge coulombic efficiency of GFC (1
6) were calculated to be approximately 480, 437, 418, 365, and 300 F g−1 at current densities of 1, 2, 5, 10, and 20 A g−1, respectively. Moreover, the charge–discharge coulombic efficiency of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) were calculated to be 61.6%, 74.4%, 91.9%, 93.6%, and 94.7% at current densities of 1, 2, 5, 10, and 20 A g−1, respectively. It is notable that the GFC composites featured excellent electrochemical performance in comparison with the literature reported Co(OH)2,28 Co(OH)2/graphene,25 and Co3O4/graphene.35,36 The performances of FC and GFC were further evaluated by Ragone plots related to energy (E) and power (P) densities (Fig. 7f). E and P are calculated by the following equations: E = (CΔV2)/2 and P = E/t, where C is the specific capacitance, ΔV is the potential window, and t is the discharge time. GFC (1
6) were calculated to be 61.6%, 74.4%, 91.9%, 93.6%, and 94.7% at current densities of 1, 2, 5, 10, and 20 A g−1, respectively. It is notable that the GFC composites featured excellent electrochemical performance in comparison with the literature reported Co(OH)2,28 Co(OH)2/graphene,25 and Co3O4/graphene.35,36 The performances of FC and GFC were further evaluated by Ragone plots related to energy (E) and power (P) densities (Fig. 7f). E and P are calculated by the following equations: E = (CΔV2)/2 and P = E/t, where C is the specific capacitance, ΔV is the potential window, and t is the discharge time. GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) could deliver the highest energy density of 38.3 W h kg−1 at a power density of 200 W kg−1 and still maintain 24 W h kg−1 at a power density of 4 kW kg−1.
6) could deliver the highest energy density of 38.3 W h kg−1 at a power density of 200 W kg−1 and still maintain 24 W h kg−1 at a power density of 4 kW kg−1.
The cycling stabilities of FC and GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) were also evaluated by GCD measurement at 1 A g−1 in 6 M KOH electrolyte (Fig. S3†). The GFC (1
6) were also evaluated by GCD measurement at 1 A g−1 in 6 M KOH electrolyte (Fig. S3†). The GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) composite electrode retained 93.5% of its initial value after 1000 cycles, whereas the capacitance of FC faded to 27.8%. The excellent cycle stability of the GFC (1
6) composite electrode retained 93.5% of its initial value after 1000 cycles, whereas the capacitance of FC faded to 27.8%. The excellent cycle stability of the GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) composite electrode could be attributed to the presence of flexible graphene, which could effectively relieve the contraction/expansion of Co(OH)2 during the consecutive charge–discharge process. The diameter of the semi-circle at high frequencies was remarkably reduced in the plot of GFC (1
6) composite electrode could be attributed to the presence of flexible graphene, which could effectively relieve the contraction/expansion of Co(OH)2 during the consecutive charge–discharge process. The diameter of the semi-circle at high frequencies was remarkably reduced in the plot of GFC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) compared with FC (Fig. S4†). This result indicated the highly reduced charge transfer resistance at the electrode/electrolyte interface caused by the graphene substrate in the composite electrode.
6) compared with FC (Fig. S4†). This result indicated the highly reduced charge transfer resistance at the electrode/electrolyte interface caused by the graphene substrate in the composite electrode.
4. Conclusions
In summary, GFC composites were prepared by a one-pot hydrothermal process. The FC with hierarchical structure was perfectly constructed using graphene sheets. The morphologies of Co(OH)2 ranged from flower petals to large flower-like microspheres and were easily controlled by changing the weight ratio of GO to cobalt salt. The GFC showed high specific capacitance as electrodes for supercapacitors, reaching 480 F g−1 at 1 A g−1 and outstanding cycling performance accompanying 93.5% capacitance, which was retained over 1000 cycles. GFC composites have promising applications for supercapacitors.Acknowledgements
This project was supported by the Shanghai Leading Academic Discipline Project (Project Number J51503), National Natural Science Foundation of China (Project Number 20976105), Shanghai Association for Science and Technology Achievements Transformation Alliance Program (Project Number LM201559), Shanghai Municipal Education Commission boosting project (Project Number 15cxy39), Science and Technology Commission of Shanghai Municipality Project (Project Number 14520503200), Shanghai Municipal Education Commission (Plateau Discipline Construction Program), Shanghai Talent Development Funding (Project Number 201335) and in part by the Scientific Research Foundation of SIT (YJ2015-35).Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- J. R. Miller and P. Simon, Science Magazine, 2008, 321, 651–652 CAS.
- B. Luo, S. Liu and L. Zhi, Small, 2012, 8, 630–646 CrossRef CAS PubMed.
- C.-C. Hu, K.-H. Chang, M.-C. Lin and Y.-T. Wu, Nano Lett., 2006, 6, 2690–2695 CrossRef CAS PubMed.
- L. Xiao, D. Wu, S. Han, Y. Huang, S. Li, M. He, F. Zhang and X. Feng, ACS Appl. Mater. Interfaces, 2013, 5, 3764–3769 CAS.
- Y. Chen, J. Wang, J. Jiang, M. a. Zhou, J. Zhu and S. Han, RSC Adv., 2015, 5, 21740–21744 RSC.
- S. Li, D. Wu, C. Cheng, J. Wang, F. Zhang, Y. Su and X. Feng, Angew. Chem., 2013, 125, 12327–12331 CrossRef.
- J. Wang, N. Yang, H. Tang, Z. Dong, Q. Jin, M. Yang, D. Kisailus, H. Zhao, Z. Tang and D. Wang, Angew. Chem., 2013, 125, 6545–6548 CrossRef.
- Y. Huang, D. Wu, S. Han, S. Li, L. Xiao, F. Zhang and X. Feng, ChemSusChem, 2013, 6, 1510–1515 CrossRef CAS PubMed.
- S. Han, J. Jiang, Y. Huang, Y. Tang, J. Cao, D. Wu and X. Feng, Phys. Chem. Chem. Phys., 2015, 17, 1580–1584 RSC.
- B. G. Choi, M. Yang, S. C. Jung, K. G. Lee, J.-G. Kim, H. Park, T. J. Park, S. B. Lee, Y.-K. Han and Y. S. Huh, ACS Nano, 2013, 7, 2453–2460 CrossRef CAS PubMed.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- L. Wang, Z. H. Dong, Z. G. Wang, F. X. Zhang and J. Jin, Adv. Funct. Mater., 2013, 23, 2758–2764 CrossRef CAS.
- U. M. Patil, M. S. Nam, J. S. Sohn, S. B. Kulkarni, R. Shin, S. Kang, S. Lee, J. H. Kim and S. C. Jun, J. Mater. Chem. A, 2014, 2, 19075–19083 CAS.
- J.-K. Chang, C.-M. Wu and I.-W. Sun, J. Mater. Chem., 2010, 20, 3729–3735 RSC.
- X.-l. Huang, X. Zhao, Z.-l. Wang, L.-m. Wang and X.-b. Zhang, J. Mater. Chem., 2012, 22, 3764–3769 RSC.
- R. Wang, X. Yan, J. Lang, Z. Zheng and P. Zhang, J. Mater. Chem. A, 2014, 2, 12724–12732 CAS.
- P. Vinothbabu and P. Elumalai, J. Solid State Electrochem., 2015, 19, 813–820 CrossRef CAS.
- A. Babaei, A. R. Taheri and M. Aminikhah, Electrochim. Acta, 2013, 90, 317–325 CrossRef CAS.
- Y. Zhang, X. Xia, J. Kang and J. Tu, Chin. Sci. Bull., 2012, 57, 4215–4219 CrossRef CAS.
- A. Jagadale, V. Kumbhar, D. Dhawale and C. Lokhande, Electrochim. Acta, 2013, 98, 32–38 CrossRef CAS.
- T. M. Higgins, D. McAteer, J. C. M. Coelho, B. M. Sanchez, Z. Gholamvand, G. Moriarty, N. McEvoy, N. C. Berner, G. S. Duesberg and V. Nicolosi, ACS Nano, 2014, 8, 9567–9579 CrossRef CAS PubMed.
- J. Chen, K. Sheng, P. Luo, C. Li and G. Shi, Adv. Mater., 2012, 24, 4569–4573 CrossRef CAS PubMed.
- J. Wang, P. Liu, Y. Huang, J. Jiang, S. Han, D. Wu and X. Feng, RSC Adv., 2014, 4, 57869–57874 RSC.
- Z. Li, J. Wang, L. Niu, J. Sun, P. Gong, W. Hong, L. Ma and S. Yang, J. Power Sources, 2014, 245, 224–231 CrossRef CAS.
- C. Zhao, X. Wang, S. Wang, Y. Wang, Y. Zhao and W. Zheng, Int. J. Hydrogen Energy, 2012, 37, 11846–11852 CrossRef CAS.
- U. Patil, S. C. Lee, J. Sohn, S. Kulkarni, K. Gurav, J. Kim, J. H. Kim, S. Lee and S. C. Jun, Electrochim. Acta, 2014, 129, 334–342 CrossRef CAS.
- D. Ghosh, S. Giri and C. K. Das, ACS Sustainable Chem. Eng., 2013, 1, 1135–1142 CrossRef CAS.
- C. Nethravathi, C. R. Rajamathi, M. Rajamathi, X. Wang, U. K. Gautam, D. Golberg and Y. Bando, ACS Nano, 2014, 8, 2755–2765 CrossRef CAS PubMed.
- Z. Liu, R. Ma, M. Osada, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2005, 127, 13869–13874 CrossRef CAS PubMed.
- S. Chen, J. Zhu and X. Wang, J. Phys. Chem. C, 2010, 114, 11829–11834 CAS.
- X. Chen, J. Cheng, Q. Shou, F. Liu and X. Zhang, CrystEngComm, 2012, 14, 1271–1276 RSC.
- J. Sampanthar and H. Zeng, Chem. Mater., 2001, 13, 4722–4730 CrossRef CAS.
- C. Yuan, X. Zhang, L. Hou, L. Shen, D. Li, F. Zhang, C. Fan and J. Li, J. Mater. Chem., 2010, 20, 10809–10816 RSC.
- C. Yuan, L. Zhang, L. Hou, G. Pang and W.-C. Oh, RSC Adv., 2014, 4, 14408–14413 RSC.
- C. Yuan, L. Yang, L. Hou, J. Li, Y. Sun, X. Zhang, L. Shen, X. Lu, S. Xiong and X. W. D. Lou, Adv. Funct. Mater., 2012, 22, 2560–2566 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00562d |
| This journal is © The Royal Society of Chemistry 2016 |