Differentiation of biothiols from other sulfur-containing biomolecules using iodide-capped gold nanoparticles†
Lvlv Jia,
Jianying Wanga,
Lei Zhua,
Yanbing Zub,
Jianfei Kong*c and
Zuofeng Chen*a
aShanghai Key Lab of Chemical Assessment and Sustainability, Department of Chemistry, Tongji University, Shanghai 200092, China. E-mail: zfchen@tongji.edu.cn
bInstitute of Bioengineering and Nanotechnology, 31 Biopolis Way, Singapore 138669
cCollege of Materials Science and Engineering, Liaoning Technical University, Fuxin, Liaoning 123000, China. E-mail: lntukjf@163.com
First published on 1st March 2016
Abstract
We describe here a simple method based on the aggregation of gold nanoparticles (GNPs) to differentiate biothiols, such as cysteine, homocysteine, cysteinylglycine and glutathione from other sulfur-containing biomolecules, such as disulfide, thioether and thiocarbonyl molecules. The GNPs are capped with iodide ions (I−), which react with the GNPs to form a chemisorbed compact layer. The aggregation of the GNPs could be induced by biothiols, but not by other sulfur-containing biomolecules and uric acid, ascorbic acid, glucose, and bovine serum albumin. The added iodide exhibits both a stabilizing effect and salt effect on the aggregation of GNPs. The significance of these two opposite effects is dependent on the concentration of iodide and the binding ability of biothiols on the GNPs. The combination of the two effects leads to quite different aggregation kinetics of GNPs from that with other halide (chloride or bromide) ions which exhibit only the salt effect. Compared to other surfactants-capped GNPs, the iodide-capped GNPs are unique with a strong and compact adsorbed layer with small steric hindrance, which accounts for the selective response towards biothiols.
Introduction
Biothiols including sulphydryl-containing amino acids such as cysteine (Cys) and homocysteine (Hcy), and small peptides such as cysteinylglycine (CysGly) and glutathione (Glu) are of importance for the clinical diagnosis of a variety of diseases.1–9 For example, Cys deficiency may be involved in liver damage, skin lesions, hair de-pigmentation, edema, lethargy, muscle and fat loss, slowed growth and AIDS.1–3 The elevated total Hcy in human plasma is a sensitive marker of cobalamin (vitamin B12) and folate deficiency, and cardiovascular disease.4 Hcy level abnormality is also related to birth defects, pregnancy complications, psychiatric disorders, and cognitive impairment in the elderly.5–7 In intracellular fluids, Glu has vital biological functions, for example, as an important antioxidant in defense against toxins and free radicals, maintenance of thiol status, and modulation of cell proliferation.8,9 The specific detection of these biothiols is not a trivial task due to their similarity in molecular structures and functional groups. Because these biothiols usually possesses a very low molar extinction coefficient, the detection of these compounds by the conventional spectrophotometric method is not effective.10 In order to overcome this problem, the derivatizaton of biothiols via the sulphydryl functionality is an option, but it complicates the analysis and increases the cost.11,12 The electrochemical method may provide a more attractive option, because it has the inherent advantages of simplicity, ease of miniaturization, high sensitivity, and relatively low cost.13–15 However, the selectivity of the electrochemical method is usually rather poor and severe detection conditions can cause fluctuating of the background currents.16The application of metal and semiconductor nanoparticles in bioanalysis has been of great interest. The analyte-induced aggregation of gold nanoparticles (GNPs) shifts the surface plasmon resonance (SPR) absorption peak toward longer wavelength, which forms the basis of colorimetric sensing techniques for a variety of molecules such as DNA,17–19 proteins,20–22 and metal ions.23–27 The interaction of amino acids with GNPs has also been investigated, and the amino acids possessing additional (besides the α-amine) functional groups such as amine, imidazole, thiol, disulfide, thioether or thiocarbonyl could induce the aggregation of GNPs.28–33 An earlier study34 utilized gold nanorods stabilized by cetyltrimethylammonium bromide (CTAB) to specifically detect sulfur-containing amino acids such as Cys, cystine, and Glu. Although gold nanorods protected by surface charges are ready to bind amino acid molecules, the interaction can be inhibited significantly when surfactant species are used to cap the nanorods; thus, the CTAB-capped gold nanorods only allow the attachment of Cys, cystine, and Glu. In another report, the nonionic fluorosurfactant (i.e. Zonyl@FSN)-capped GNPs was used for the direct colorimetric analysis and postcolumn detection of Hcy and Cys.35,36 At the same time, the nonionic surfactant Brij 35-capped GNPs was also developed as a reactive postcolumn reagent for the determination of Hcy, Cys, CysGly, Glu and methionine.37 Although the functional groups other than sulfur-containing groups could be excluded from interacting with GNPs, all these methods34–38 are not capable of differentiation of biothiols including the sulphydryl-containing amino acids and peptides, from disulfide amino acids, thioether amino acid, as well as thiocarbonyl molecules. It is thus important to develop more diverse capping ligands to stabilize GNPs and achieve desirable sensing selectivity. Studies that attempt to correlate the sensing selectivity with the nature of the capping agents are consequently worthwhile.
Halide species can adsorb either irreversibly or reversibly on metal electrodes such as platinum and gold, which has been extensively studied with combining techniques of thin-layer electrochemistry, low-energy electron diffraction, Auger electron spectroscopy, X-ray photoelectron spectroscopy and others.39–44 These chemisorbed halide layers can withstand rinsing with water and electrolyte solution with the adsorption strength in the sequence I− > Br− > Cl−. While Br− and Cl− adsorbed less intensely in their anionic form, I− was reported to react with the metal electrodes to form a compact uncharged adsorbed layer (i.e., I atom).42–44 In contrast to platinum, halides anions are stable complexants for soluble gold species.45,46 The anodic dissolution of a gold electrode in aqueous solution thus can be enhanced by added halide ions and the thermodynamics of various Au–halide–H2O systems have been summarized.45,46 The different adsorption behaviors of these halide species on noble metal electrodes have led to quite different electrochemical behaviors, for example, in electrogenerated chemiluminescence (ECL) with aliphatic amines as coreactant, much stronger ECL emission was observed at the iodide-modified Pt or Au electrodes than those with chloride or bromide, because the more intense adsorption of iodide species at Pt or Au electrodes can greatly retard the formation of surface oxides, leading to more facile oxidation of aliphatic amines.47 An earlier report has also shown the crucial role of iodide ions in the shape control of the seed-mediated NPs synthesis because it can strongly and selectively bind to the Au(111) facet and favor the formation of anisotropic structures.48,49 Although there is no direct evidence on the curved gold at the nanoscale, a similar adsorption behavior of iodide species is expected on the GNPs surface as on the bulk gold surface. We speculate that the compact chemisorbed layer of iodide species on GNPs may suppress the interaction between GNPs and some biomolecules leading to selective detection of other biomolecules.
Here, we synthesized water soluble GNPs capped with iodide and studied the interaction of the iodide-capped GNPs with a variety of biomolecules including 20 standard amino acids, cystine, Hcy, homocystine, CysGly, Glu, Gluox (oxidized glutathione), uric acid (UA), ascorbic acid (AA), glucose, and bovine serum albumin (BSA). It has been found that only sulphydryl-containing amino acids and peptides could induce GNPs aggregation leading to obvious color change of the colloidal solutions. Interestingly, the capping agent also exhibits a significant salt effect (i.e. NaI acts as a salt) and the kinetics of the colorimetric evolution of the colloidal solutions induced by biothiols could be tuned quite different by simply varying the concentration of added iodide. The selective response of the iodide-capped GNPs towards biothiols was ready to extend to the non-biological thiol molecules. The comparison of the iodide-capped GNPs with those capped with the conventional surfactants revealed that the high selectivity towards thiols may be attributable to the strong and compact adsorption and the small steric hindrance effect of iodide species on the GNPs surface. To our knowledge, this is the first report on the monoatomic species used as a capping agent for nanoparticles in colorimetric sensing. This study may also be helpful to achieve a better understanding of the important role played by the capping agents by correlating the sensing selectivity with the nature of the capping agents.
Experimental
Chemicals
Hydrogen tetrachloroaurate(III) trihydrate (HAuCl4·3H2O), trisodium citrate, 20 standard amino acids, cystine, homocysteine, homocystine, cysteinylglycine, glutathione, oxidized glutathione, cysteamine, cystamine, 2-mercaptoethanol, 2-mercaptoethylamine, 6-mercapto-1-hexanol, uric acid, ascorbic acid, glucose, bovine serum albumin, Zonyl FSN (F(CF2CF2)1–7CH2CH2O(CH2CH2O)0–15H), and Brij 35 (polyoxyethyleneglycol dodecyl ether) were purchased from Sigma-Aldrich. 2,2′-Thiodiethanol, thiocarbamide (i.e., thiourea), thiocarbohydrazide, halide salts including NaCl, NaBr and NaI were obtained from Alfa Aesar. Other chemicals were analytical reagent graded and used as received. All solutions were prepared with deionized water (Mili Q, Millipore).Apparatus
Transmission electron microscopy (TEM) images were taken using a Philips microscope (Tecnai 20) operated at an acceleration voltage of 200 kV. UV-vis spectra were recorded on a Hewlett-Packard 8453 diode-array UV-vis spectrophotometer using quartz cuvettes with an optical path length of 1 cm at room temperature. The electrochemical measurements were performed on a CHI 760B electrochemical workstation (Chenhua Instruments, Shanghai). The three-electrode system consisted of a working electrode, a saturated calomel reference electrode (SCE), and a coiled Pt wire counter electrode. The solution pH for electrochemical measurements was adjusted to 3 which was close to pH of the GNPs colloidal solution.Gold nanoparticle synthesis
Colloidal GNPs with average diameters of 40 nm were prepared following the literature procedure.28 All glassware used for preparation of gold colloids was thoroughly washed with freshly prepared aqua regia (HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl = 1
HCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), rinsed extensively with deionized and ultrahigh purity water sequentially, and then dried in an oven at 100 °C for 2–3 h. A 60 mL solution of 0.01% HAuCl4 was brought to a vigorous boil with stirring in a round-bottom flask fitted with a reflux condenser, and then 0.12 mL 5.0% sodium citrate was added to the stirring and refluxing HAuCl4 solution (for the preparation of smaller-sized GNPs, larger amount of sodium citrate was used accordingly). The solution was maintained at the boiling point with continuous stirring for about 15 min. After the solution was allowed to cool to room temperature with continued stirring, 0.1 mM NaI were added to cap the GNPs without any purification pretreatment unless stated otherwise. The colloidal solution buffered by the remnant citrate has a pH of ∼3.0 and was used without adjustment unless stated otherwise. Assuming spherical particles and density equivalent to that of bulk gold (19.30 g cm−3), the concentration of the GNPs was calculated to be ∼0.148 nM (note ESI†). The TEM specimens were prepared by depositing an appropriate amount of GNPs or the iodide-capped GNPs onto the carbon-coated copper grids, and excess solution was wicked away by a filter paper. The grid was subsequently dried in air.
3), rinsed extensively with deionized and ultrahigh purity water sequentially, and then dried in an oven at 100 °C for 2–3 h. A 60 mL solution of 0.01% HAuCl4 was brought to a vigorous boil with stirring in a round-bottom flask fitted with a reflux condenser, and then 0.12 mL 5.0% sodium citrate was added to the stirring and refluxing HAuCl4 solution (for the preparation of smaller-sized GNPs, larger amount of sodium citrate was used accordingly). The solution was maintained at the boiling point with continuous stirring for about 15 min. After the solution was allowed to cool to room temperature with continued stirring, 0.1 mM NaI were added to cap the GNPs without any purification pretreatment unless stated otherwise. The colloidal solution buffered by the remnant citrate has a pH of ∼3.0 and was used without adjustment unless stated otherwise. Assuming spherical particles and density equivalent to that of bulk gold (19.30 g cm−3), the concentration of the GNPs was calculated to be ∼0.148 nM (note ESI†). The TEM specimens were prepared by depositing an appropriate amount of GNPs or the iodide-capped GNPs onto the carbon-coated copper grids, and excess solution was wicked away by a filter paper. The grid was subsequently dried in air.
Results and discussion
Characterization of the halide-capped gold nanoparticles
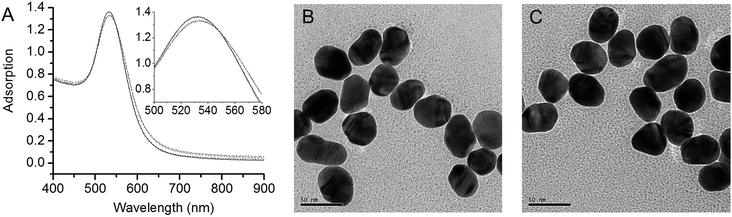
40 nm GNPs with deep-red in color have been synthesized in aqueous solution by reduction of HAuCl4 with citrate. Fig. 1A shows the SPR adsorption spectra of 40 nm GNPs capped with different halide ions. The GNPs stabilized electrostatically by the adsorbed citric anions exhibited a SPR absorption peak at a wavelength of 533 nm. The spectral band was nearly unchanged upon the addition of NaCl and NaBr up to 15 mM. Further increasing the amount of NaCl or NaBr would make GNPs less stable due to the salt effect. By contrast, the SPR peak shifts toward longer wavelength by around 2 nm upon addition of only 0.1 mM NaI. Similar phenomenon was reported previously by the binding of long chain thiols and surfactants to GNPs.50 The spectral shift with adsorbed capping agents may be attributed to the increase of average refractive index of the environment surrounding of the GNPs. Since the adsorption of iodide species at the gold surface is much stronger than that with chloride and bromide species (see ESI, Fig. S1†), a compact adsorbed layer on GNPs surface is expected only with iodide, which leads to a more distinct variation on the environment surrounding of GNPs. Negligible changes on spectra were observed upon further addition of NaI from 0.1 to 15 mM, indicating that a complete covering of GNPs by the iodide species has been achieved above 0.1 mM NaI. The TEM images in Fig. 1B and C show that the GNPs are generally spherical and well dispersed, and exhibit no difference before and after iodide species capping. The iodide-capped GNPs (0.1 mM NaI) exhibited good stability in aqueous solutions with no SPR spectral changes observed after being stored at 4 °C for at least one week.Interaction of the iodide-capped gold nanoparticles with biomolecules
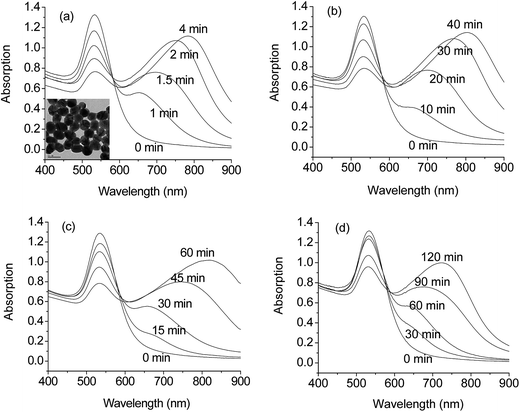
We examined the optical absorption changes of the colloidal solution of the iodide-capped GNPs upon the addition of a variety of biomolecules, including 20 standard amino acids, cystine, Hcy, homocystine, CysGly, Glu, Gluox, UA, AA, glucose, and BSA. Fig. 2 shows that upon the addition of 5 μM HCy, Cys, CysGly and Glu, the absorption bands at 535 nm decreased with new bands appeared at longer wavelengths, suggesting that the GNPs are ready to bind these biothiols and aggregate subsequently (e.g., see TEM images in Fig. 2a inset). The aggregation kinetics of iodide-capped GNPs was found to be in the sequence of Hcy > Cys > CysGly > Glu, which is generally consistent with that using GNPs stabilized electrostatically by the adsorbed citric anions30 or GNPs capped with nonionic fluorosurfactants.35,36By contrast, little spectral change occurred in the presence other biomolecules (i.e. except for the four biothiols mentioned above) even at much higher concentrations (∼100 μM). It is particularly interesting to note that the rapid response of GNPs toward disulfide amino acids (homocystine, cystine and Gluox) and thioether amino acid (methionine) was completely inhibited when GNPs were capped with the iodide species, as shown in Fig. 3. NaCl and NaBr were also used to cap the GNPs, and their responses towards the biomolecules were examined. No selectivity was observed for the chloride and bromide-capped GNPs, i.e., besides biothiols, the disulfide amino acids and the thioether amino acid could also induce solution color change. Apparently, the high selectivity of the iodide-capped GNPs in response to the biothiols (Scheme 1) is attributable to the unique feature of the adsorbed iodide species.
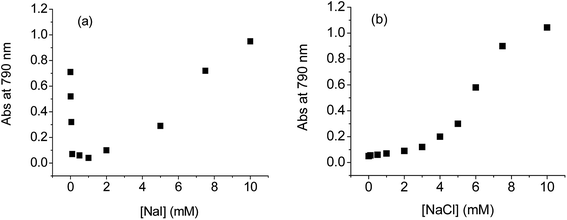
The inhibiting effect of the iodide species on disulfide amino acids and thioether amino acid greatly depends on the iodide concentration. Fig. 4 shows the dependence of the extinction changes of the colloidal GNPs solutions on NaI concentration upon the addition of a large amount (100 μM) of homocystine. A rapid increase of the absorbance at 790 nm was observed for the colloidal solution in the absence of any iodide species. With addition of increasing amounts of NaI, the solution color change became greatly retarded, indicating a slowed aggregating rate of GNPs. In the presence of 0.1 mM NaI, the colloidal solution did not respond towards 100 μM homocystine for at least 12 h, indicating that a complete covering of GNPs was achieved by the iodide species at this concentration. Although the aggregation kinetics induced by the cystine, Gluox, or methionine was relatively more sluggish, a similar amount of NaI was generally required in order to achieve a complete covering of GNPs for a complete inhibition of the aggregation. The results indicated that these disulfide amino acids and thioether amino acid were not able to replace the pre-adsorbed iodide species and attach to the GNPs surface. Further increase in NaI concentration up to 15 mM also result in no colorimetric evolution by added homocysteine, cystine, Gluox, or methionine.
 | ||
| Fig. 4 The effect of NaI concentration on the time courses of extinction (at 790 nm) of 40 nm GNPs upon the addition of 100 μM homocystine. | ||
To demonstrate the selective response of the iodide-capped GNPs towards diverse thiols, some small non-biological thiol molecules, such as cysteamine, 2-mercaptoethanol, 2-mercaptoethylamine and 6-mercapto-1-hexanol were examined, Fig. S2.† As can be seen, these molecules (∼5 μM) were also able to induce the color change of the colloidal solution in the presence of 0.1 mM NaI. Like biothiols, the added non-biological thiols could replace the adsorbed iodide and attach to the GNPs, rendering the colloidal solution less stable and aggregating. On the other hand, some small non-biological disulfide, thioether and thiocarbonyl molecules, such as cystamine, 2,2′-thiodiethanol, thiourea and thiocarbohydrazide were also examined, Fig. S3.† All these molecules can induce rapid aggregation of the 40 nm GNPs stabilized electrostatically by the adsorbed citric anions. However, in the presence of 0.1 mM NaI, the colloidal solution did not respond towards these molecules (∼100 μM) for at least 12 h.
Biothiols-induced nanoparticles aggregation
The aggregations of the iodide-capped GNPs induced by biothiols (as well as non-biological thiols) molecules were found to be affected by several factors. Experimental conditions, such as solution ionic strength (adjusted by added NaI), pH and GNPs size could significantly influence the aggregation process.Fig. 5a shows the effect of NaI concentration on the extinction change of the colloidal GNPs solution at 790 nm upon the addition of 5 μM Glu. It can be seen that the aggregating rate of GNPs denoted by the extinction change is significantly dependent on NaI concentration. Generally, at a low level of NaI concentration, the aggregating rate of GNPs decreases with increasing NaI concentration. The result is consistent with that observed in Fig. 4. Under the condition of low ionic strength, the well-known cross-linking mechanism may account for the aggregation, where the amino acid molecules bound to the NPs act as cross-linking agents to establish connection between nanoparticles via either hydrogen bonding or electrostatic interaction.28,34 The increase of NaI concentration in this case would increase the difficulty for the biothiol molecules to attach on the GNPs as biothiols have to compete with the pre-adsorbed iodide species. A minimum absorbance at 790 nm was observed as NaI concentration reaching ∼1 mM, indicating a lowest aggregating rate of GNPs at this NaI concentration.
With further increasing NaI concentration, however, the aggregation process of GNPs was accelerated with increasing NaI concentration. It is well known that the GNPs stabilized electrostatically by adsorbed citric anions were very sensitive to the salt concentration.51 The increase of the salt concentration would screen the electrostatic repulsion between GNPs, which makes the GNPs less stable and in favor of the aggregation. For the iodide-capped GNPs, the salt effect would also become prominent as NaI concentration increases to a certain extent. Under the high-salt condition, we believe that the replacement of some iodide species on GNPs by the biothiol molecules makes the colloidal solution unstable, and the aggregation could be driven by the London–van der Waals attraction force. The high-salt induced GNPs aggregation has been reported in a previous study,19 where the GNPs were stabilized by single-strand DNA molecules and rapid aggregation could be induced by DNA hybridization in high-salt solutions.
The above observation is significantly different from that with added chloride (or bromide). As shown in Fig. 5b, the increase of NaCl concentration (or NaBr concentration) monotonously accelerated the aggregation of GNPs in the presence of Glu, indicating that only salt effect was exhibited in the presence of chloride or bromide. Besides Glu, similar results were also obtained for other biothiols induced aggregation in terms of these halides as capping agents.
As shown in Table 1, the NaI concentration that requires to achieving the lowest aggregating rate varies with different biothiols. The variation could be connected with the different binding abilities of these biothiols on the GNPs. The binding of biothiols, which makes the colloidal solution less stable, may first involve the replacement of pre-adsorbed iodide species on the GNPs. In this case at low concentrations of iodide ions (i.e., on the left side of the extremum point), the iodide species acts mainly as stabilizing agents. However, at high concentrations of iodide ions (i.e., on the right side of the extremum point), the salt effect may exceed the stabilizing effect, and the aggregation process is accelerated. Therefore, the appearance of the extremum point at a low concentration of NaI, such as the case of Glu, indicates that the salt effect of the iodide ions was dominant in a wide concentration range. Since the stabilizing effect which resulted from the adsorption of iodide species is ready to be overwhelmed by the salt effect, this may further imply a relatively stronger adsorption of Glu on the GNPs. On the contrary, the appearance of the extremum point at a high concentration of NaI, such as the cases of Hcy and Cys, may reflect a relatively less intense binding ability of these biothiols. The different adsorption abilities of these biothiols are related with their structural chain lengths. Previous reports on the self-assembly monolayers suggested that molecules with longer structural chains may adsorb more intensely on the gold substrate.52,53 In addition, the adsorption strength of these biothiols on GNPs is well consistent with the electrochemical result for the same biothiols adsorbed at a polycrystalline gold electrode, Fig. S4.†
| Biothiols | Cys | Hcy | CysGly | Glu |
|---|---|---|---|---|
| [NaI] (mM) | ∼12 | ∼10 | ∼4 | ∼1 |
The effect of the GNPs size on the colorimetric evolution was also investigated. Generally, the iodide-capped GNPs with smaller size (e.g., 12 nm) would aggregate more rapidly towards biothiols. Although the aggregation kinetics induced by the biothiols is dependent on the GNPs size, the colorimetric response of the GNPs towards disulfide, thioether, or thiocarbonyl molecules was still completely suppressed in the presence of iodide species. Interestingly, the minimum amount of NaI that required to completely inhibit the response of disulfide, thioether, or thiocarbonyl molecules was varied with the GNPs size. For GNPs of 12 nm, a minimum NaI of 0.5 mM was required to achieve the complete inhibition in comparison to 0.1 mM NaI for 40 nm GNPs. This result may be at least partially rationalized by the different specific surface area that needs to be covered for GNPs of different sizes.
Comparison of the iodide- and surfactant-capped gold nanoparticles
In comparison with the commonly used capping agents such as fluorosurfactants, Brij 35, and CTAB, which are generally toxic, or some of their environmental safeties have not been fully tested, sodium iodide is commonly available and environment-friendly. Based on the above observations, the iodide-capped GNPs are capable of differentiating biothiols from other sulfur-containing biomolecules, and the major results are summarized in Tables 2 and 3. The highly selective colorimetric response of the iodide-capped GNPs towards biothiols and non-biological thiols is unique and striking. Some previous studies indicated that amino acids could be attached to GNPs via their α-amine groups or additional functional groups such as amine, imidazole, thiol, disulfide, thioether or thiocarbonyl.28–33 Therefore, the amino acid molecules possessing one of these additional functional groups could serve as a cross-linking agent for GNPs. However, the experimental results obtained in this study revealed different behaviors between biothiols/non-biological thiols and disulfide/thioether/thiocarbonyl molecules in interacting with the iodide-capped GNPs. Compared with other species, biothiols/non-biological thiols could be attached to the GNPs via the strong Au–S bond, which renders the binding more thermodynamically favorable. Although disulfide/thioether/thiocarbonyl molecules might also form Au–S linkages with the uncovered gold substrate, all of these species were hindered from interacting with the iodide-capped GNPs. This is because the surface of the GNPs is covered by a layer of iodide species, and the dissociation of disulfide/thioether/thiocarbonyl molecules prior to their adsorption on GNPs is a metal surface-coupled process52,54–56 which is not available in the presence of a compact chemisorbed layer of iodide species.| Biomolecules | Homocysteine (Hcy) | Cysteine (Cys) | Glutathione (reduced, Glu) | Cysteinylglycine (CysGly) | Homocystine | Cystine | Glutathione (oxidized, Gluox) | Methionine |
|---|---|---|---|---|---|---|---|---|
| a R, R1, and R2 represent the organic groups.b “✓” represents “aggregation”, and “✗” represents “non-aggregation”. | ||||||||
| Sulfur forma | R–SH | R–SH | R–SH | R–SH | R1–S–S–R2 | R1–S–S–R2 | R1–S–S–R2 | R1–S–R2 |
| 40 nm GNPsb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 40 nm I-GNPsb | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ |
| Non-biological molecules | Cysteamine | 2-Mercaptoethanol | 2-Mercaptoethylamine | 6-Mercapto-1-hexanol | Cystamine | 2,2′-Thiodiethanol | Thiourea | Thiocarbohydrazide |
|---|---|---|---|---|---|---|---|---|
| a R, R1, and R2 represent the organic groups.b “✓” represents “aggregation”, and “✗” represents “non-aggregation”. | ||||||||
| Sulfur forma | R–SH | R–SH | R–SH | R–SH | R1–S–S–R2 | R1–S–R2 | R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S S |
| 40 nm GNPsb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 40 nm I-GNPsb | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ |
The earlier study35,36 has demonstrated the selective colorimetric sensing of Hcy and Cys by using the fluorosurfactant (FSN)-capped GNPs; while biothiols with a longer structural chain such as CysGly and Glu were non-responsive in addition to the disulfide and thioether amino acids. The electrochemical experiment in Fig. S5a† revealed very close desorption peak potentials and coulombic charges of iodide and FSN species at the polycrystalline gold electrode, indicating a close adsorption strength and coverage for both species at the gold substrate. We speculate that the different response of iodide- and FSN-capped GNPs towards CysGly and Glu may be attributed to the different steric hindrance effect of iodide and FSN species. The large biothiol molecules might be hindered from interacting with GNPs by the adsorbed bulky FSN layer, while these biothiols might easily access the surface of iodide-capped GNPs and compete with iodide species to adsorb on the GNPs.
Although it is also a bulky capping agent, Brij 35-capped GNPs exhibited a wider response where, besides the biothiols, the thioether amino acid could also induce a rapid aggregation.37 The electrochemical experiment in Fig. S5b† revealed a relatively small and broad desorption peak for Brij 35 on the polycrystalline gold electrode as comparing to that with iodide or FSN species, indicating the formation of a looser and disordered adsorbed layer for Brij 35 on the gold surface. We believe that the adsorbed Brij 35 layer could not block all the active sites of GNPs for the dissociative-adsorption of the thioether, and the aggregation is thus induced. Based on the discussion above, Scheme 2 illustrates clearly the important role played by the capping species on the binding selectivity.
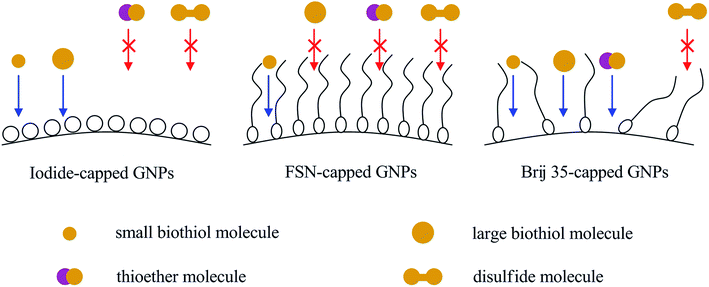 | ||
| Scheme 2 Schematic picture showing the I-, FSN-, and Brij 35-capped GNPs and the binding selectivity. | ||
Since iodide might form an uncharged (i.e. I atom) adsorbed layer species on the metal surface, we speculate that iodine (I2) may also serve as a sulphydryl-responsive capping agent. This is the case. The selectivity of the iodine-capped GNPs was the same as that of the iodide-capped ones, except that the response kinetics was relatively sluggish with no ionic strength effect resulted from the added capping reagent itself, i.e., the response kinetics of the GNPs towards thiols monotonously decreased with increasing the amount of added iodine with no salt effect. Nevertheless, the disadvantages of the direct iodine capping were obvious. Compared to iodide, iodine is sublimed, chromatic and much less soluble in aqueous solution, making the sensing sophisticated and inaccurate.
Conclusions
Water soluble GNPs capped with iodide species have been synthesized and characterized. Examination of their interactions with a variety of biomolecules revealed that the aggregation of the iodide-capped GNPs could be induced by sulphydryl-containing amino acids and peptides, but not by other amino acids including sulfur-containing ones like disulfide and thioether, UA, AA, glucose, and BSA. The experimental results revealed that iodide ions exhibit not only stabilizing effect but also salt effect on the colorimetric evolution of the colloidal solutions, which is dependent on the concentration of the added iodide and the competitive adsorption of iodide species and biothiol molecules on the GNPs. In this way, the colorimetric evolution of the colloidal solutions induced by biothiols could be tuned quite different by simply varying the concentration of the added iodide. The same selectivity was observed for the iodide-capped GNPs toward the sulfur-containing non-biological molecules. In comparison with the conventional surfactant-capped GNPs, the selective response of the iodide-capped GNPs towards thiols could be attributed to the strong and compact adsorbed layer and the small steric hindrance effect of iodide species. This study may find application as a postcolumn reagent for assay of biothiols in high-performance liquid chromatography.Acknowledgements
Z.-F. C. thanks the National Natural Science Foundation of China (21405114, 21573160), The Recruitment Program of Global Youth Experts by China, and Science & Technology Commission of Shanghai Municipality (14DZ2261100) for support.References
- S. Shahrokhian, Anal. Chem., 2001, 73, 5972 CrossRef CAS PubMed.
- W. Wang, O. Rusin, X. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15949 CrossRef CAS PubMed.
- W. Droge, H. P. Eck and S. Mihm, Immunol. Today, 1992, 13, 211 CrossRef CAS PubMed.
- S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostino, P. W. F. Wilson and P. A. Wolf, N. Engl. J. Med., 2002, 346, 476 CrossRef CAS PubMed.
- P. M. Ueland and S. E. Vollset, Clin. Chem., 2004, 50, 1293 Search PubMed.
- V. Cavalca, G. Cighetti, F. Bamonti, A. Loaldi, L. Bortone, C. Novembrino, M. De Franceschi, R. Belardinelli and M. D. Guazzi, Clin. Chem., 2001, 47, 887 CAS.
- O. Nygard, S. E. Vollset, H. Refsum, I. Stensvold, A. Tverdal, J. E. Nordrehaug, M. Ueland and G. Kvave, J. Am. Vet. Med. Assoc., 1995, 274, 1526 CrossRef CAS.
- H. J. Forman, H. Zhang and A. Rinna, Mol. Aspects Med., 2009, 30, 1 CrossRef CAS PubMed.
- D. M. Townsend, K. D. Tew and H. Tapiero, Biomed. Pharmacother., 2003, 57, 145 CrossRef CAS.
- G. Chwatko and E. Bald, Talanta, 2000, 52, 509 CrossRef CAS PubMed.
- N. Adam and J. R. Kramer, Aquat. Geochem., 1999, 5, 1 CrossRef.
- L. Gallo-Martinez, A. Sevillano-Cabeza, P. Campins-Falco and F. Bosch-Reig, Anal. Chim. Acta, 1998, 370, 115 CrossRef CAS.
- Z. Chen, H. Zhen, C. Lu and Y. Zu, Langmuir, 2007, 23, 10816 CrossRef CAS PubMed.
- L. P. Wu, J. Li and H. M. Zhang, Electroanalysis, 2015, 27, 1195 CrossRef CAS.
- S. G. Ge, M. Yan, J. J. Lu, M. Zhang, F. Yu, J. H. Yu, X. R. Song and S. L. Yu, Biosens. Bioelectron., 2012, 31, 49 CrossRef CAS PubMed.
- O. Chailapakul, P. Aksharanandana, T. Frelink, Y. Einaga and A. Fujishima, Sens. Actuators, B, 2001, 80, 193 CrossRef CAS.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078 CrossRef CAS PubMed.
- J. J. Storhoff, A. A. Lazarides, R. C. Mucic, C. A. Mirkin, R. L. Letsinger and G. C. Schatz, J. Am. Chem. Soc., 2000, 122, 4640 CrossRef CAS.
- K. Sato, K. Hosokawa and M. Maeda, J. Am. Chem. Soc., 2003, 125, 8102 CrossRef CAS PubMed.
- J.-M. Nam, S.-J. Park and C. A. Mirkin, J. Am. Chem. Soc., 2002, 124, 3820 CrossRef CAS PubMed.
- C. M. Niemeyer, Angew. Chem., Int. Ed., 2001, 40, 4128 CrossRef CAS.
- S. Mann, W. Shenton, M. Li, S. Connolly and D. Fitzmaurice, Adv. Mater., 2000, 12, 147 CrossRef CAS.
- J.-W. Liu and Y. Lu, J. Am. Chem. Soc., 2003, 125, 6642 CrossRef CAS PubMed.
- S. O. Obare, R. E. Hollowell and C. J. Murphy, Langmuir, 2002, 18, 10407 CrossRef CAS.
- S.-Y. Lin, S.-W. Liu, C.-M. Lin and C.-H. Chen, Anal. Chem., 2002, 74, 330 CrossRef CAS PubMed.
- S.-Y. Lin, C.-H. Chen, M.-C. Lin and H.-F. Hsu, Anal. Chem., 2005, 77, 4821 CrossRef CAS PubMed.
- Y. Kim, R. C. Johnson and J. T. Hupp, Nano Lett., 2001, 1, 165 CrossRef CAS.
- Z.-Y. Zhong, S. Patskovskyy, P. Bouvrette, J. H. T. Luong and A. Gedanken, J. Phys. Chem. B, 2004, 108, 4046 CrossRef CAS.
- K. Naka, H. Itoh, Y. Tampo and Y. Chujo, Langmuir, 2003, 19, 5546 CrossRef CAS.
- F. X. Zhang, L. Han, L. B. Israel, J. G. Daras, M. M. Maye, N. K. Ly and C.-J. Zhong, Analyst, 2002, 127, 462 RSC.
- P. R. Selvakannan, S. Mandal, S. Phadtare, R. Pasricha and M. Sastry, Langmuir, 2003, 19, 3545 CrossRef CAS.
- Z. J. Li, X. J. Zheng, L. Zhang, R. P. Liang, Z. M. Li and J. D. Qiu, Biosens. Bioelectron., 2015, 68, 668 CrossRef CAS PubMed.
- H. L. Gao, W. W. Shen, C. Lu, H. Liang and Q. P. Yuan, Talanta, 2013, 115, 1 CrossRef CAS PubMed.
- P. K. Sudeep, S. T. S. Joseph and K. G. Thomas, J. Am. Chem. Soc., 2005, 127, 6516 CrossRef CAS PubMed.
- C. Lu, Y. Zu and V. W. W. Yam, Anal. Chem., 2007, 79, 666 CrossRef CAS PubMed.
- C. Lu and Y. Zu, Chem. Commun., 2007, 3871 RSC.
- C. Lu, Y. Zu and V. W. W. Yam, J. Chromatogr., A, 2007, 1163, 328 CrossRef CAS PubMed.
- F. Ghasemi, M. R. Hormozi-Nezhad and M. Mahmoudi, Anal. Chim. Acta, 2015, 882, 58 CrossRef CAS PubMed.
- M. W. Breiter, Electrochim. Acta, 1963, 8, 925 CrossRef.
- W. Bold and M. W. Breiter, Electrochim. Acta, 1961, 5, 145 CrossRef.
- D. M. Novak and B. E. Conway, J. Chem. Soc., Faraday Trans., 1981, 77, 2341 RSC.
- R. F. Lane and A. T. Hubbard, J. Phys. Chem., 1975, 79, 808 CrossRef CAS.
- J. F. Rodriguez, J. E. Harris, M. E. Bothwell, T. Mebrahtu and M. P. Soriaga, Inorg. Chim. Acta, 1988, 148, 123 CrossRef CAS.
- G. M. Berry, M. E. Bothwell, B. G. Bravo, G. J. Cali, T. Mebrahtu, S. L. Michelhaugh, J. F. Rodriguez and M. P. Soriaga, Langmuir, 1989, 5, 707 CrossRef CAS.
- G. H. Kelsall, N. J. Welham and M. A. Diaz, J. Electroanal. Chem., 1993, 361, 13 CrossRef CAS.
- M. A. Diaz, G. H. Kelsall and N. J. Welham, J. Electroanal. Chem., 1993, 361, 25 CrossRef CAS.
- Y. B. Zu and A. J. Bard, Anal. Chem., 2000, 72, 3223 CrossRef CAS PubMed.
- A. Rai, A. Singh, A. Ahmad and M. Sastry, Langmuir, 2006, 22, 736 CrossRef CAS PubMed.
- T. H. Ha, H.-J. Koo and B. H. Chung, J. Phys. Chem. C, 2007, 111, 1123 CAS.
- Y.-G. Sun and Y.-N. Xia, Anal. Chem., 2002, 74, 5297 CrossRef CAS PubMed.
- J. Zhou, D. A. Beattie, J. Ralston and R. Sedev, Langmuir, 2007, 23, 12096 CrossRef CAS PubMed.
- Z. Y. Li, S. C. Chang and R. S. Williams, Langmuir, 2003, 19, 6744 CrossRef CAS.
- K. A. Peterlinz and R. Georgiadis, Langmuir, 1996, 12, 4731 CrossRef CAS.
- M. Ozcan, F. Karadag and I. Dehri, Colloids Surf., A, 2008, 316, 55 CrossRef.
- E. E. Oguzie, Y. Li and F. H. Wang, J. Colloid Interface Sci., 2007, 310, 90 CrossRef CAS PubMed.
- B. Monterroso-Marco and B. Lopez-Ruiz, Talanta, 2003, 61, 733 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Cyclic voltammograms of halide-, FSN-, Brij 35-, and biothiols-modified polycrystalline gold electrodes; UV-vis spectra of 40 nm iodide-capped GNPs in the presence of a variety of sulfur-containing non-biological molecules. See DOI: 10.1039/c6ra00451b |
| This journal is © The Royal Society of Chemistry 2016 |