Microwave-assisted synthesis of void-induced graphene-wrapped nickel oxide hybrids for supercapacitor applications†
Abstract
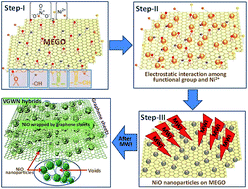
Here we demonstrate a simple strategy for the large-scale synthesis of void-induced and graphene-wrapped nickel oxide (VGWN) hybrids using domestic microwave irradiation. When a homogeneously mixed aqueous suspension of partially microwave exfoliated graphene oxide (MEGO) sheets and nickel nitrate nanoparticles is irradiates with a microwave, the in situ formation of voids with wrapping of NiO nanoparticles with graphene sheets is easily realized. The novel VGWN hybrid materials were used for investigation of electrochemical capacitive behaviours. Remarkably, such hybrid structure provides the right combination of electrode properties for high-performance supercapacitors and cyclic stability. The wrapping of graphene sheets on the NiO nanoparticles can offer highly conductive pathways by bridging individual NiO nanoparticles together, thus facilitating the charge/discharge rate and cycling performance of supercapacitors. The hybrid materials displayed enhanced capacitive performance (549 F g−1 at 10 mV s−1). Additionally, over 88% of the initial capacitance was retained after repeating the cyclic voltammetry test for 2500 cycles. The enhanced electrochemical performance can be ascribed to the synergic effects of the two components' voids and wrapping, suggesting VGWN hybrids as novel electrode materials promising potential applications as high-performance energy storage devices.

 Please wait while we load your content...
Please wait while we load your content...