Pickering emulsions stabilized by shape-controlled silica microrods
Fangli Lou,
Lishaya Ye,
Miqiu Kong,
Qi Yang,
Guangxian Li and
Yajiang Huang*
College of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering of China, Sichuan University, Chengdu 610065, China. E-mail: hyj@scu.edu.cn
First published on 26th February 2016
Abstract
Silica microrods with aspect ratios (AR) varying from 1 to 16 but similar surface chemical characteristics are synthesized and their potential in preparing stable oil-in-water Pickering emulsions is explored. The stability of hexadecane/water emulsions is found to strongly depend on the AR and concentration of particles. Emulsions stabilized with these silica microrods are quiescently stable for quite a long period of time (over months), while emulsions with spherical particles of similar diameters destabilize after only dozens of hours. The superior stabilization efficiency of microrods with larger ARs is attributed to their higher steric hindrance, interface adsorption energy and capillary forces.
Introduction
Emulsions consisting of two immiscible fluids are usually thermodynamically instable. Low molar mass surfactants or emulsifiers are often required to prevent catastrophic coalescence in these systems. As a versatile alternative to conventional amphiphilic surfactants, micro- or nanoscale solid particles have proved their extraordinary capability in sterically stabilizing emulsions. Unlike molecular surfactants, these particles can be trapped at the fluid–fluid interface irreversibly and facilitate the formation of so-called Pickering emulsions with long-term stability.1,2 The superior stabilization effect of solid particles stems from the very high free energy barrier ΔE that a interfacial particle must overcome to detach from a fluid–fluid interface toward one of the bulk phases,3 which is usually several orders of magnitude higher than the thermal energy kT.4 It is to a large extend due to their superior stability that Pickering emulsions have been of a revived interest in the synthesis of new materials especially for health-related fields such as food, pharmaceutical and cosmetics applications in the past decade.5Hitherto, tremendous efforts have been made aiming to reveal the decisive role of particle size,6 concentration,7 wettability,8,9 particle–particle interaction,10 addition of surfactants or electrolytes,11,12 interfacial rheology,13 as well as flow14,15 on the formation and stability of Pickering emulsions. The particles utilized include precipitated colloids,8 polymer latexes,6 clays,16 and fumed particles,7,15 which are usually of diverse shapes (such as spheroids, ellipsoids, needles/cylinders, sheets, or irregular fractal structures). However, when one compares the relative role of different factors which determine the stability of emulsions, it is usually very difficult to exclude the contribution coming from the discrepancy in the particle shape.
Shape-anisotropic particles such as microrods receive relatively scarce attention in the field of Pickering emulsions although interesting packing and orientation behavior at planar fluid interfaces or in bulk fluids due to their shape have been revealed extensively.17–21 Compared with spherical particles, the most noticeable advantage of anisotropic microrods is the distinct decrease in the structural or rheological percolation threshold, which usually leads to improved mechanical strengths at lower particle loadings. Another important property of microrods is the strong attractive capillary interaction between them when immersed in fluids.17,18 To minimize the total distortion energy of fluid interface between two neighboring microrods, strong capillary interaction arises, forcing them approach each other or form long-range tip-to-tip or side-to-side structures.19,20,22
It is only recently that the crucial role of shape anisotropy of particles on emulsion stability has received adequate attention.14,23–28 Due to the anisotropy in particle shape, effectively limited droplet coalescence and higher emulsion volumes could be achieved in Pickering emulsions at lower interfacial coverages.27,29 These morphology changes are closely correlated with the particular long-range self-assembly and orientation behavior of anisotropic particles, which improve the rigidity and viscoelasticity of particle-laden interfaces significantly. Anisotropic particles used in present studies include metal oxide particles,14,23 polymer particles23 and cellulose nanocrystals.25,27 However, the preparation process of these particles is usually complicated and inefficient, and the surface chemical properties or aspect ratios are sometimes difficult to be controlled. Therefore, there is an urgent need of exploring the key role of aspect ratio in stabilizing Pickering emulsions by using model anisotropic particles with explicit surface chemistries and controllable aspect ratios.
Recently, the exciting advances in colloidal synthesis enable the precise control of particle shape and surface properties. Herein, silica microrods with variable aspect ratios ranging from 1 to 16 but similar surface chemistry are prepared using a one-pot synthesis method proposed by Kuijk et al.30 by simply changing the synthesis temperature. The effects of aspect ratio and concentration of these microrods on the stability of hexadecane-in-water Pickering emulsions are then examined. The phenomena observed are discussed in terms of the packing and orientation of these microrods at the fluid–fluid interface.
Materials and methods
Preparation of spherical particles
Monodisperse SiO2 microspheres were synthesized in a flask using a modified Stöber method31 as follows. Firstly, 16.25 mL of ethanol, 24.75 mL of de-ionized double distilled water and 9.0 mL of ammonium hydroxide solution (NH4OH, 25–28% NH3 in water) were mixed at 1100 rpm at room temperature. Secondly, 4.5 mL of tetra-ethyl orthosilicate (TEOS, 98%, ACROS Organics) and 45.5 mL of ethanol were added rapidly into the mixture. After stirring for about 1 min, the stirring speed was reduced to 400 rpm. Finally, the flask was sealed by parafilm and the reaction was left for 2 h at room temperature to form silica particles. The particles were cleaned by consecutive centrifuging, decanting, and redispersing in ethanol for 5 times.Preparation of silica microrods
High-quality silica microrods with various aspect ratios (AR) were prepared using an emulsion-based method developed by Kuijk et al.30 Firstly, 30 g of polyvinylpyrrolidone (PVP, Mn = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Sigma-Aldrich) was dissolved in 300 mL of n-pentanol by sonication in a 500 mL beaker. 30 mL of ethanol, 8.4 mL of double distilled water and 2 mL of 0.18 mol L−1 sodium citrate dihydrate solution (99%, Alfa Aesar) in water were then added orderly and the resultant mixture was stirred to an uniform solution. Secondly, 6.75 mL of ammonia and 3 mL of TEOS were added in sequence and then the mixture was stirred again. Finally, the sealed beaker was placed in a temperature humidity chamber for 24 h. The AR of microrods was tuned by changing the temperature in the range of 10–30 °C. After consecutive centrifuging (4000 rpm), decanting, and redispersing in ethanol and water for 2 times, the final suspension was then centrifuged 3 times at 2800 rpm for 15 min to remove small rods and improve monodispersity. The resultant silica microrods were dried overnight in a vacuum freeze dryer and then were stored in dry form.
000 g mol−1, Sigma-Aldrich) was dissolved in 300 mL of n-pentanol by sonication in a 500 mL beaker. 30 mL of ethanol, 8.4 mL of double distilled water and 2 mL of 0.18 mol L−1 sodium citrate dihydrate solution (99%, Alfa Aesar) in water were then added orderly and the resultant mixture was stirred to an uniform solution. Secondly, 6.75 mL of ammonia and 3 mL of TEOS were added in sequence and then the mixture was stirred again. Finally, the sealed beaker was placed in a temperature humidity chamber for 24 h. The AR of microrods was tuned by changing the temperature in the range of 10–30 °C. After consecutive centrifuging (4000 rpm), decanting, and redispersing in ethanol and water for 2 times, the final suspension was then centrifuged 3 times at 2800 rpm for 15 min to remove small rods and improve monodispersity. The resultant silica microrods were dried overnight in a vacuum freeze dryer and then were stored in dry form.
Characterization of silica particles
The size of particles was measured using scanning electron microscopy (SEM, JEOLSJM-5900VL). As the roughness of particle tablets made from micron-size particles has a significant impact on the contact angle measurement, the surface chemistries of dried particles were accessed by Fourier transform infrared spectroscopy (FTIR) in transmittance mode on a Nicolet 6700 FTIR spectrometer. The spectra were collected in the 4000–650 cm−1 range at a resolution of 4 cm−1.Emulsion preparation
Oil in water (o/w) emulsions were prepared by mixing hexadecane and a SiO2 particle aqueous suspension at an oil/aqueous phase ratio of 30/70 and a total volume of 10 mL. All the particle concentrations mentioned were based on the aqueous phase. Certain amount of particles were dispersed in 7 mL double distilled water in a glass sample vial by sonication for 3 min and then 3 mL chemically pure hexadecane was added into the milky suspensions obtained. Finally, the mixture was sonicated for 2 min to facilitate migration of silica particles toward the interface and ensure sufficient emulsification. The resultant emulsions were vacuumed to remove residual bubbles. To determine the effect of particle aspect ratio on emulsion formulation and stability, a series of aqueous SiO2 suspensions with a constant particle concentration of 1.0 wt% but different ARs were prepared. When investigating the effect of concentration, particles with AR = 10 ± 1.0 and 16 ± 1.5 were used and the particle concentration varied from 0% to 3.5 wt%.Characterization of emulsions
All samples were allowed to equilibrate over time through limited coalescence to reach their final droplet size distribution. An optical microscope (BX51, Olympus) equipped with a long working distance objective (50×) and a Pixelfly CCD camera was used to visualize the size distribution of emulsion droplets over time and the packing and organization of particles at the interface. The emulsion droplet size was determined by image analysis. Approximately 200 droplets were analyzed for each sample. The number-averaged diameter (DN) and volume-averaged diameter (DV) of droplets were calculated by eqn (1),
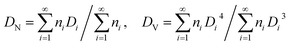 | (1) |
To infer the packing and orientation of silica rods at the hexadecane/water interface, some styrene-in-water emulsions were also prepared in view of the similarity of hexadecane and styrene in surface tension (27 mN m−1 and 32 mN m−1, respectively)27 and the polymerizability of styrene. 3.5 mL aqueous suspensions containing 1.0 wt% silica rods of different ARs were sonicated for 3 min and degassed with nitrogen for 10 min, and then 1.5 mL styrene with 2,2-azobisisobutyronitrile AIBN (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w, radical polymerization initiator) was added. After sonicated for 2 min, the resultant emulsions were degassed again with nitrogen gas for 10 min before polymerization at 60 °C for 24 h. After precipitated and washed by centrifugation, a few droplets of resultant bead suspensions were dripped onto a Millipore 0.2 μm pore filter paper to obtain the sediment by vacuum filtration and the filter paper were used for SEM characterization.
1 w/w, radical polymerization initiator) was added. After sonicated for 2 min, the resultant emulsions were degassed again with nitrogen gas for 10 min before polymerization at 60 °C for 24 h. After precipitated and washed by centrifugation, a few droplets of resultant bead suspensions were dripped onto a Millipore 0.2 μm pore filter paper to obtain the sediment by vacuum filtration and the filter paper were used for SEM characterization.
Surface coverage (C)
The surface coverage (C) determined from the amount of particles added in the emulsion and the oil volume effectively trapped within the droplets, and the latter can be deduced directly from the mean oil droplet size.32 Assuming that all particles are adsorbed at the surface of oil droplets, the interface area that the particles may cover is estimated from their equatorial section Seq = nπd2/4 = 3mp/2dρ for spheres and Seq = ndl = 4mp/πdρ for rods, where n is the total number of particles, mp is the total mass of particles used, d and l is the diameter and length of particles, respectively, and ρ is the density of particles taken as 2.2 g cm−3. The total interfacial area Sint of the emulsion directly linked to the average droplet size DN can be expressed as Sint = 6Vd/DN, where Vd is the oil volume. The surface coverage C, defined as C = Seq/Sint can thus be estimated after measuring the drop size DN:
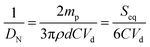 | (2) |
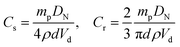 | (3) |
Results and discussion
Morphology and surface chemistry of silica microrods
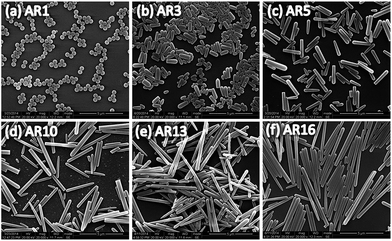
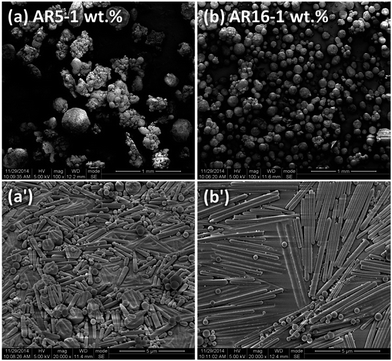
Fig. 1 shows the SEM images of silica microspheres or microrods synthesized and Table 1 summarizes corresponding dimensions of these particles determined. The spherical Stöber particles have an averaged diameter of ∼0.36 μm. For microrods synthesized from the Kuijk's method, as the reaction temperature elevated from 10 °C to 30 °C, the diameter of rod-like particles decreases slightly from ∼0.38 μm to ∼0.31 μm. The length of these particles shows higher sensitivity to the synthesis temperature. The aspect ratio (AR) increases significantly from 3 ± 0.4 to 16 ± 1.5 with increasing temperature, accompanying a slight increase in the polydispersity. According to the Kuijk's method,30 the hydrophilic hydrolysates of silica precursor TEOS dissolve in water-rich droplets, thereby supplying hydrolyzed TEOS for the condensation growth of microrods from silica nucleus at the surface of water/hexadecane emulsions. The diameter of microrods is then fixed by the size of water droplets in the water-in-hexadecane emulsions. Therefore, the slight decrease in the diameter of microrods with temperature observed here can be interpreted presumably by the smaller size of water droplets at higher synthesis temperatures. Raising the reaction temperature will lead to an increase in growth rate but a decrease in nucleation, thus elongated microrods can be obtained. Since the particles nucleate successively and the growth process is very soon at higher temperatures, the earlier nucleation sites start growing prior to the later ones and consume TEOS hydrolysates inside water droplets more rapidly until the depletion of silicon source. Consequently, the absolute polydispersity of AR also increases with the temperature.| Particle | TSynthesis (°C) | l (μm) | d (μm) | Aspect ratio (AR) |
|---|---|---|---|---|
| a Sample AR1 and AR3–AR16 were synthesized by using the Stöber method and the Kuijk method, respectively. | ||||
| AR1 | 25 | 0.36 | 0.36 | 1 ± 0.1 |
| AR3 | 10 | 1.13 | 0.38 | 3 ± 0.4 |
| AR5 | 15 | 1.94 | 0.37 | 5 ± 0.5 |
| AR10 | 20 | 3.90 | 0.36 | 10 ± 1.0 |
| AR13 | 25 | 4.76 | 0.35 | 13 ± 1.2 |
| AR16 | 30 | 5.09 | 0.31 | 16 ± 1.5 |
No organic group is found in the FTIR spectra of silica particles synthesized (Fig. 2a), demonstrating the hydrophilicity nature of these particles. To quantitatively evaluate the concentration of hydrophilic groups, the hydroxyl index (IOH, 3450 cm−1) and silanol index (ISi-OH, 950 cm−1) were calculated. The corresponding peak intensities were normalized to the intensity of reference peak at 800 cm−1 which corresponds to Si–O stretching vibrations and almost does not change under all conditions. Fig. 2b proves that the surfaces of all synthesized silica particles possess similar hydrophilicities regardless of the change in the synthesis method or in the synthesis temperature. Hereafter, the silica particles prepared are used as model particles to reveal the influence of AR of particles on the stability of Pickering emulsions.
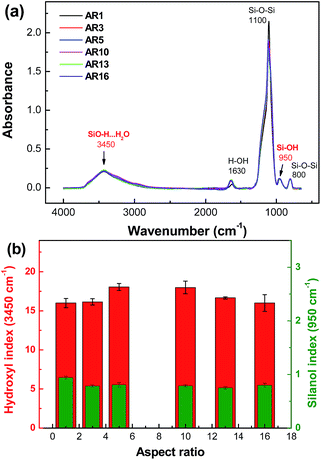 | ||
| Fig. 2 (a) FTIR spectra and (b) hydroxyl index (3450 cm−1) and silanol index (950 cm−1) of silica microrods with various ARs. | ||
Effect of aspect ratio of particles
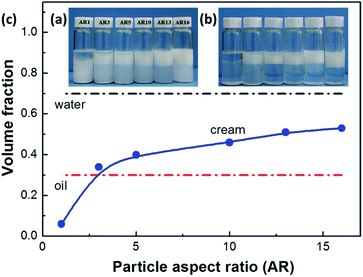
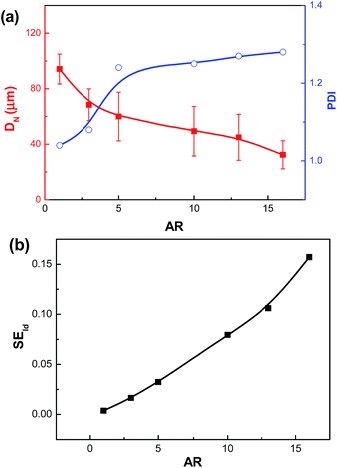
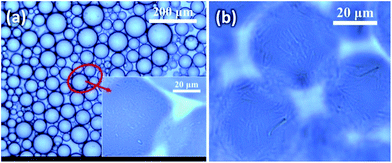
Fig. 3a and b shows the photographs of hexadecane-in-water emulsions stabilized with 1 wt% silica particles of various aspect ratios (AR) after prepared for 1 day and 30 days. The sample containing silica spheres (AR = 1) partially phase-separates after 1 day into an aqueous silica suspension at the bottom of the vial and a transparent oil phase on the top of it (Fig. 3a). Only a little emulsion phase can be seen on the topside, implying the poor capability of these silica spheres in making Pickering emulsions. After stored for 30 days (Fig. 3b), the oil–water emulsion phase separates completely. All silica spheres, including those in the aqueous suspension, seem to settle to the bottom, leading to the formation of a clear water phase. On the contrary, samples with microrods generate much stable oil-in-water emulsions on the top of vial (Fig. 3a), which are stable over months (Fig. 3b). To probe the nature of continuous phase in the emulsion created, a small volume of cream is dripped onto a glass slide coated with a layer of water. It was found that the cream spread on the surface of water layer and tiny oil droplets formed the top, indicating that the continuous phase of emulsion should be water and the droplet phase should be oil. Increasing the AR of particles generated more emulsified cream phase (Fig. 3a and c). These results suggest that both the volume and stability of emulsified creams strongly depend on the AR of particles. And these longer microrods could be trapped stably within the emulsions. As long as the AR of particles reaches 3, stable solid-stabilized emulsions can be generated. Similar phenomena have also been found in decane-in-water emulsions stabilized by microsized spindle-like hematite particles but with a higher critical AR value of 4.6.23Fig. 4a shows the average size and polydispersity index (PDI = DV/DN) of oil droplets of Pickering emulsions stabilized with 1.0 wt% microrods of different ARs. With increasing AR, the droplet size decreases monotonically from 94.2 μm for AR = 1 ± 0.1 to 32.4 μm for AR = 16 ± 1.5, together with an expanded emulsified phase volume as shown in Fig. 3. Meanwhile, the polydispersity of emulsion droplet size also increases with AR, especially when AR ≤ 5 ± 0.5. To compare the stabilization efficiency of particles with various ARs, the ratio of particle length to droplet size (SEld) is calculated. Fig. 4b shows that the particle stabilization efficiency SEld exhibits a nearly exponential growth with AR. The SEld value increases from 3.8 × 10−3 for AR = 1 ± 0.1 to 1.56 × 10−1 for AR = 16 ± 1.5 with a 41-fold variation. Therefore, the microscopic morphology of emulsions also evidences that the stabilization efficiency of particles depends largely on their ARs.
Effect of particle concentration
The influence of particle concentration is considered in Pickering emulsions stabilized with microrods of AR = 10 ± 1.0 but of different concentrations (0–3.5 wt%) stored for 1 day and 30 days after emulsification. The sample free of particles is found to phase-separate into oil phase and water phase in a few days rather than form stable emulsions, and no stable droplets are observed by the optical microscope on the day 30. Upon the addition of microrods of AR = 10 ± 1.0, emulsions that can exist stably over months are obtained. Fig. 5 shows the volume of emulsion phase increases steadily with increasing particle concentration, indicating that more aqueous phase is entrapped into the oil phase. A maximum volume fraction of emulsions of 60 vol% is obtained at a particle concentration of 2.5 wt%, beyond which the emulsion volume remains almost constant.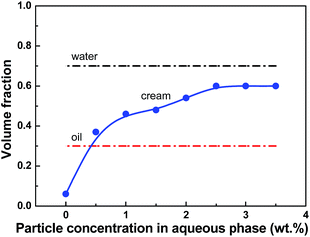 | ||
| Fig. 5 Emulsion volume of hexadecane-in-water Pickering emulsions stabilized with particles of AR = 10 ± 1.0 with different concentrations 1 day after emulsification. | ||
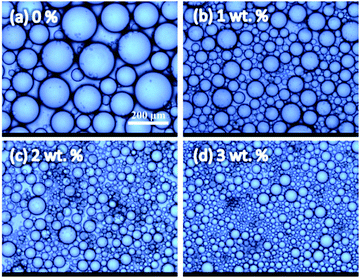
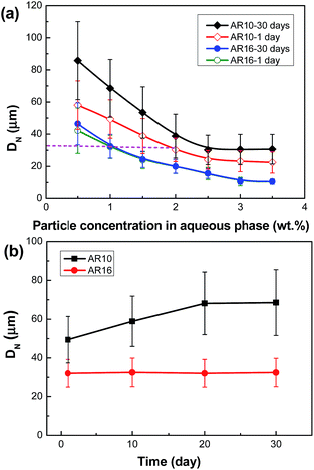
Fig. 6 shows the optical micrographs of emulsions stabilized with silica microrods of various concentrations 1 day after the preparation. The droplet diameter decreases with the particle concentration increases up to 2.5 wt% and remains almost constant after that, which is consistent with the change in the macroscopic volume of emulsions (Fig. 5). Fig. 7a compares the dependence of droplet size on particle concentration for emulsion stabilized by silica particles with two different ARs. For two kinds of samples, the droplet size becomes smaller with increasing particle concentration at first and then levels off at different concentrations. The critical concentration is 2.5 wt% for AR10 particles, whereas it increases to 3–4 wt% for AR16 particles. The long-term stabilization efficiency of silica microrods also shows a strong dependence on their concentration and aspect ratio although totally water–oil separation is not observed in these particle-stabilized emulsions. As shown in Fig. 7a and b, emulsions stabilized with AR10 particles experience obvious coalescence within 20 days, leading to a detectable shrinkage in the emulsion volume with time (Fig. 5). Intriguingly, this coarsening process happens even when exceeding the critical concentration. For instance, the droplet size of emulsion stabilized by 3 wt% AR10 particles grows from 23.2 μm to 30.5 μm after 30 day storage with an increase about 31%. For emulsions stabilized with 0.5 wt% AR16 particles, a marginal coalescence with droplet size increasing by only ∼10% is observed. Emulsions with higher AR16 loadings show extremely excellent stability over time and no coalescence can be observed even after been stored for a month.
Packing and orientation of interfacial rods
The abovementioned results imply that the structural stability of Pickering emulsions is highly sensitive to the aspect ratio of solid particles incorporated. For emulsions stabilized with spherical silica particles, no stable emulsion can be made. Though emulsions stabilized with short silica rods exhibit improved short-term stability, noticeable coalescence still can be detected during long-term storage even when exceeding their critical particle concentrations. For emulsion stabilized with longer silica rods, smaller droplets are obtained and no obvious coalescence is observed during long storage duration.To reveal the underlying mechanism for these phenomena, the organization behavior of silica rods at the fluid interface of emulsions should be elucidated at first. Fig. 8 shows the optical micrographs of droplet surface in Pickering emulsions stabilized with 1 wt% AR5 and AR16 particles. For both emulsions, rod-armoured droplet surface is displayed and silica rods are scarcely found in the water matrix phase. Therefore, Fig. 8 proves that silica rods are all mainly trapped at the oil–water interface. For AR16-stabilized emulsion, some long rods appear between adjacent droplets (Fig. 8b). However, due to the limited resolution of optical microscopy, the detail organization of silica rods is still difficult to be discriminated.
To further infer the discrepancy in the packing and orientation of silica rods at the hexadecane-in-water emulsion interface, some styrene (St)-in-water emulsions stabilized by 1 wt% AR5 and AR16 silica rods were prepared in view of the similarity of hexadecane and St in the surface tension (27 mN m−1 and 32 mN m−1, respectively)27 and the polymerizability of St. St droplets were solidified through polymerization after emulsification, thereby enabling a detail visualization of individual droplets by using the conventional SEM technique. As shown in Fig. 9a and b, despite the solidification of St droplets, the dependence of droplet size on the AR of silica microrods shares a similar tendency with that observed in hexadecane-in-water emulsions by the optical microscopy observation. Few tiny polystyrene (PS) particles formed during polymerization are also observed in these SEM images. For rod-like particles in Fig. 9a′ and b′, the bound state in side-by-side and parallel orientation seem to be the most stable configuration. It is because shape-anisotropic particles can deform the interface thus inducing capillary attractions between particles to force them approaching each other and packing more orderly.17,20,33 Stable oblique configurations are feasible as well, especially for shorter particles. A close inspection of Fig. 9a′ reveals that although the packing of AR5 rods on the surface of droplets is denser than AR16 rods, it is somewhat more random. For AR16 stabilized droplets (Fig. 9b′), some droplet surface regions are even fully free of silica rods. The arrangement of these long silica rods exhibits higher localized regularity presumably due to their stronger capillary forces.17,20,23 However, network structure between droplets stabilized by cellulosic nanorods with ultrahigh aspect ratios (∼130) as found by Capron et al.27 is not observed in this study.
Theoretically, if the concentration of interfacially located particles in a Pickering emulsion is insufficient to completely cover all the fluid interfaces, continuous coalescence will take place to reduce the total specific surface area until a surface saturation of densely packed particles is reached.34 Fig. 10a shows the surface coverages of emulsions stabilized by 1 wt% particles with different ARs estimated from their average droplet sizes (Fig. 4a). Interestingly, the surface coverage is found to be a monotonically decreasing function of AR of silica rods. In this sense, emulsions stabilized by AR16 rods should be more feasible to coalescence, which is contrary to the experimental results presented here. A lower surface coverage is directly related to the decreased droplet size at higher ARs of rods which consequently increases the total interfacial area.27 Another relevant reason may be the locally inhomogeneous distribution of longer silica rods at the curved droplet surface (Fig. 9) due to the stronger capillary attraction forces between them. The surface coverage of droplets generally increases with the particle concentration (Fig. 10b). However, the coverage ratio of droplets stabilized with the shorter AR10 rods is more sensitive to the change in the particle concentration than that stabilized with the longer AR16 rods.
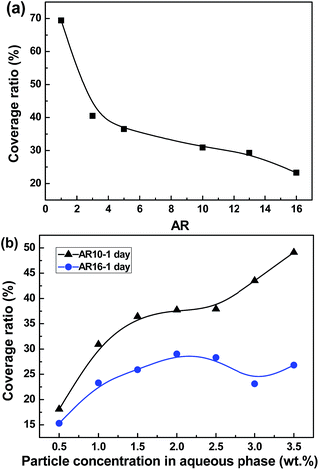 | ||
| Fig. 10 Dependence of estimated coverage percentage of droplet surface on (a) the AR where 1.0 wt% particles introduced in the aqueous phase and (b) the particle concentration. | ||
Fig. 11 shows the evolution of the average inverse droplet diameter (1/D) of emulsions stabilized with AR10 and AR16 as a function of the total equatorial surface area (Seq/Vd) of rods. At low total equatorial surface areas (also low rod concentrations), good linear dependence can be found between 1/D and Seq/Vd for both kinds of emulsions. This linear dependence is in agreement with the prediction of limited coalescence process2 and confirms the preferential adsorption of silica rods at the oil–water interface. The comparison of Fig. 10 and 11 shows that when shorter rods are involved, a higher surface coverage will be obtained with a smaller slope in the 1/D vs. Seq/Vd curve. This linearity of the curve holds as long as the limited coalescence of droplets is still effective.27 The sample with AR16 rods displays a large deviation from this linear relationship at 3.0 wt%, indicating that the rod concentration is in excess and adding more rods will no longer affect the droplet size. At that time, the droplet size will depend mainly on the intensity of mechanical energy putted into the emulsions via sonicating or stirring.2 Similar phenomenon can also be found in the curve of AR10 which shows a deviation at 2.5 wt%.
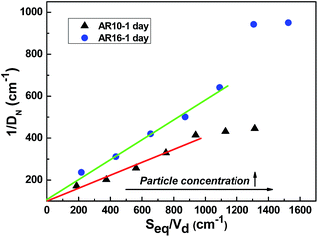 | ||
| Fig. 11 Average inverse droplet diameter (1/DN) of hexadecane-in-water emulsions as a function of the total equatorial surface area of particles for emulsions stabilized with AR10 and AR16 particles. | ||
The reason for the coalescence observed beyond the critical particle concentration in rod-stabilized Pickering emulsions during long-term storage is likely relevant to the desorption of microrods due to gravity. Although it has been suggested that the gravitational force can be neglected for colloids of less than 10 μm in size,27 the aggregation of these micron-sized particles with a higher density than two liquid phases may make the gravity nonnegligible and lead to coalescence due to the desorption of particles from the fluid interface. However, as shown in Fig. 9, longer AR16 rods prone to pack more compactly on the surface of droplets due to stronger capillary attractions and therefore should lead to more pronounced gravity effect and desorption process. Actually, the interface adsorption energy of particles is also affected by the ARs. It is suggested that particles with larger ARs possess higher interface adsorption energy35 and thus are less likely to desorb from the fluid/fluid interface. Furthermore, the steric hindrance of rod particles, which also depends on the magnitude of interface adsorption energy,36 should not be neglected. In this sense, the interface adsorption energy and the steric hindrance of AR16 rods play a dominate role over the gravity effect, thereby leading to stronger inhibition of droplet coalescence.36
Based on the above experimental results and discussions, a schematic diagram for illustrating the packing and orientation of particles with different ARs at the fluid/fluid interface is depicted in Fig. 12 below. Due to the stronger capillary forces, the longer rods pack more orderly but inhomogeneously. Longer rods and their aggregates exhibit higher space steric hindrance at the interface due to their larger size, thereby conferring to the emulsion higher resistance to coalescence even at lower particle concentrations. As a result, coalescence and phase separation are arrested, resulting in the formation of kinetically stable Pickering emulsions. On the contrary, shorter particles arrange randomly but uniformly on the droplet surface, and meanwhile are more likely to desorb from the interface.
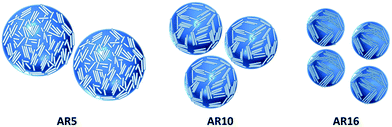 | ||
| Fig. 12 Schematic illustration for the effect of particle aspect ratio on the surface coverage and droplet size of Pickering emulsion. | ||
Conclusions
High-quality and monodisperse silica microrods with similar surface chemistry but diverse aspect ratios (AR) are successfully synthesized with an emulsion-based method. These micron-sized silica rods with aspect ratios larger than 2–3 can effectively stabilize the hexadecane-in-water emulsion. Emulsions stabilized with longer particles acquired smaller droplet size and expanded emulsified volume, demonstrating their superior stabilization efficiencies because of higher steric hindrance and interface adsorption energy. Similar results are observed when increasing the particle concentration while keeping a fixed particle AR, implying that stable emulsions can be obtained at lower particle loadings by utilizing particles with higher ARs. Although the surface coverage is higher for shorter particles, the coalescence still occurred beyond the critical particle concentration, which is likely relevant to the desorption of microrods due to gravity. Contrarily, longer rods can be trapped at fluid/fluid interface more stably and create higher steric hindrance against droplet coalescence regardless of their lower interfacial coverage. As a consequence, it is not necessary to totally cover the fluid/fluid interface for suppressing droplet coalescence and in preparing a stable emulsion.Acknowledgements
We are grateful to the financial support from the National Natural Science Foundation of China (51373109, 51503133, 51421061), Sichuan Youth Science and Technology Foundation (2015JQ0012), and the State Key Laboratory of Polymer Materials Engineering (Grant No. sklpme2014-3-07).Notes and references
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156 CrossRef CAS
.
- S. U. Pickering, J. Chem. Soc., Trans., 1907, 91, 2001 RSC
.
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 100, 503 CrossRef
.
- P. Pieranski, Phys. Rev. Lett., 1980, 45, 569 CrossRef CAS
.
- Y. Chevalier and M.-A. Bolzinger, Colloids Surf., A, 2013, 439, 23 CrossRef CAS
.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2001, 17, 4540 CrossRef CAS
.
- B. P. Binks, J. Philip and J. A. Rodrigues, Langmuir, 2005, 21, 3296 CrossRef CAS PubMed
.
- B. P. Binks and C. P. Whitby, Colloids Surf., A, 2005, 253, 105 CrossRef CAS
.
- B. P. Binks, L. Isa and A. T. Tyowua, Langmuir, 2013, 29, 4923 CrossRef CAS PubMed
.
- T. N. Hunter, R. J. Pugh, G. V. Franks and G. J. Jameson, Adv. Colloid Interface Sci., 2008, 137, 57 CrossRef CAS PubMed
.
- C. P. Whitby, D. Fornasiero and J. Ralston, J. Colloid Interface Sci., 2009, 329, 173 CrossRef CAS PubMed
.
- B. P. Binks and S. O. Lumsdon, Phys. Chem. Chem. Phys., 1999, 1, 3007 RSC
.
- D. Tambe and M. Sharma, Adv. Colloid Interface Sci., 1994, 52, 1 CrossRef CAS
.
- S. Vandebril, J. Vermant and P. Moldenaers, Soft Matter, 2010, 6, 3353 RSC
.
- Q. Li, Y. J. Huang, S. T. Xi, Q. Yang and G. X. Li, AIChE J., 2013, 59, 4373 CrossRef CAS
.
- Y. Nonomura and N. Kobayashi, J. Colloid Interface Sci., 2009, 330, 463 CrossRef CAS PubMed
.
- E. Van Nierop, M. Stijnman and S. Hilgenfeldt, Europhys. Lett., 2005, 72, 671 CrossRef CAS
.
- L. Botto, E. P. Lewandowski, M. Cavallaro and K. J. Stebe, Soft Matter, 2012, 8, 9957 RSC
.
- B. Madivala, J. Fransaer and J. Vermant, Langmuir, 2009, 25, 2718 CrossRef CAS PubMed
.
- J.-C. Loudet, A. M. Alsayed, J. Zhang and A. G. Yodh, Phys. Rev. Lett., 2005, 94, 18301 CrossRef CAS PubMed
.
- E. P. Lewandowski, J. A. Bernate, A. Tseng, P. C. Searson and K. J. Stebe, Soft Matter, 2009, 5, 886 RSC
.
- D. Kim, W. D. Kim, M. S. Kang, S.-H. Kim and D. C. Lee, Nano Lett., 2015, 15, 714 CrossRef CAS PubMed
.
- B. Madivala, S. Vandebril, J. Fransaer and J. Vermant, Soft Matter, 2009, 5, 1717 RSC
.
- W. Zhou, J. Cao, W. Liu and S. Stoyanov, Angew. Chem., Int. Ed., 2009, 48, 378 CrossRef CAS PubMed
.
- I. Kalashnikova, H. Bizot, B. Cathala and I. Capron, Langmuir, 2011, 27, 7471 CrossRef CAS PubMed
.
- P. Wongkongkatep, K. Manopwisedjaroen, P. Tiposoth, S. Archakunakorn, T. Pongtharangkul, M. Suphantharika, K. Honda, I. Hamachi and J. Wongkongkatep, Langmuir, 2012, 28, 5729 CrossRef CAS PubMed
.
- I. Kalashnikova, H. Bizot, P. Bertoncini, B. Cathala and I. Capron, Soft Matter, 2013, 9, 952 RSC
.
- J. W. de Folter, E. M. Hutter, S. I. Castillo, K. E. Klop, A. P. Philipse and W. K. Kegel, Langmuir, 2014, 30, 955 CrossRef CAS PubMed
.
- S. V. Daware and M. G. Basavaraj, Langmuir, 2015, 31, 6649 CrossRef CAS PubMed
.
- A. Kuijk, A. van Blaaderen and A. Imhof, J. Am. Chem. Soc., 2011, 133, 2346 CrossRef CAS PubMed
.
- W. Stober, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef
.
- M. Destribats, V. Lapeyre, M. Wolfs, E. Sellier, F. Leal-Calderon, V. Ravaine and V. Schmitt, Soft Matter, 2011, 7, 7689 RSC
.
- E. P. Lewandowski, M. Cavallaro Jr, L. Botto, J. C. Bernate, V. Garbin and K. J. Stebe, Langmuir, 2010, 26, 15142 CrossRef CAS PubMed
.
- S. Arditty, C. P. Whitby, B. P. Binks, V. Schmitt and F. Leal-Calderon, Eur. Phys. J. E, 2003, 11, 273 CrossRef CAS PubMed
.
- L. Dong and D. T. Johnson, Langmuir, 2005, 21, 3838 CrossRef CAS PubMed
.
- E. Dickinson, Curr. Opin. Colloid Interface Sci., 2010, 15, 40 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |