Synthesis of porphyrin sensitizers with a thiazole group as an efficient π-spacer: potential application in dye-sensitized solar cells†
Abstract
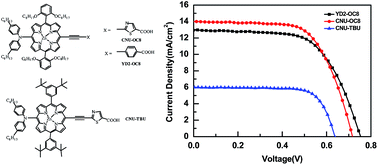
Herein, we report porphyrin sensitizers for DSSCs, coded CNU-OC8 and CNU-TBU, which were synthesized using a donor–π-bridge–acceptor approach. The porphyrin sensitizers were subjected to electrochemical experiments to study their electron distribution, intramolecular charge transfer and HOMO–LUMO levels. The optical and photovoltaic properties of these synthesized porphyrins were measured and compared with those of the YD2-OC8 benchmark dye. To further characterize, we simulated the electrochemical and optical properties of the dyes, which are perfectly in agreement with the experimental data. The new CNU-OC8 and CNU-TBU porphyrin sensitizers provided power conversion efficiencies of 6.49% and 3.19%, respectively, compared to a conversion efficiency of 6.10% for YD2-OC8 under similar conditions. These results indicate that CNU-OC8 exhibits better photovoltaic performance than the benchmark YD2-OC8 sensitizer in a liquid I−/I3− redox electrolyte.

 Please wait while we load your content...
Please wait while we load your content...