An innovation for development of Erlenmeyer–Plöchl reaction and synthesis of AT-130 analogous: a new application of continuous-flow method†
Abstract
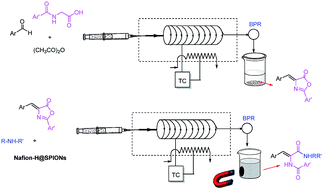
The development of eco-friendly and efficient processes via one-pot multicomponent synthesis is a very attractive topic. In this work, the Erlenmeyer–Plöchl azlactone synthesis was carried out through unique, safe, fast and practical conditions without any catalyst, applying a simple microreactor and gave the corresponding products exclusively. A continuous, first microflow synthesis of N-benzoylglycine carbamide derivatives as AT-130 analogues catalyzed by Nafion-H@SPIONs was also established successfully.

 Please wait while we load your content...
Please wait while we load your content...