Thoroughly mesoporous TiO2 nanotubes prepared by a foaming agent-assisted electrospun template for photocatalytic applications†
Abstract
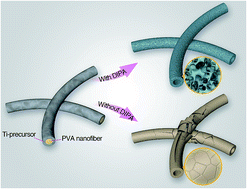
A thoroughly mesoporous long TiO2 nanotube with intact morphology was firstly prepared using a foaming agent-assisted electrospun template method in which an electrospun water-soluble PVA nanofiber was used as the template. The large volumes of vapors released from the introduced foaming agents are attributed to the production of mesopores with uniform spatial distribution on the whole TiO2 tube wall and these mesopores prevent damage to the TiO2 nanotubular morphology during the PVA template removal. The present work represents a critically important solution in advancing the electrospinning technique for generating mesoporous nanotubes in an effective and facile manner.

 Please wait while we load your content...
Please wait while we load your content...