Development of an antibody-binding modular nanoplatform for antibody-guided targeted cell imaging and delivery†
Hansol Kim‡
,
Young Ji Kang‡,
Junseon Min,
Hyeokjune Choi and
Sebyung Kang*
Department of Biological Sciences, School of Life Sciences, Ulsan National Institute of Science and Technology (UNIST), Ulsan, Korea. E-mail: sabsab7@unist.ac.kr; Fax: +82-52-217-5309; Tel: +82-52-217-5325
First published on 10th February 2016
Abstract
A polyvalent antibody-binding lumazine synthase protein cage nanoparticle (ABD–AaLS) is constructed by genetically fusing lumazine synthase and antibody-binding domains. ABD–AaLS effectively captures targeting antibodies in an orientation-controlled manner by selectively binding to the Fc region of antibodies derived from a variety of species, such as rabbits, rats, and mice, on demand by simple molecular recognition. The resulting antibody/ABD–AaLS non-covalent complexes specifically recognize and bind to their target cells in vitro, guided by antibodies displayed on the surface of ABD–AaLS. ABD–AaLS has an additional internal cavity and exterior sites for encapsulation and attachment of cargo molecules such as drugs and diagnostic probes. ABD–AaLS effectively serves as a universal antibody-binding nanoplatform to display various targeting antibodies on demand through molecular recognition as well as to acquire additional functionalities without altering the essential properties of the targeting antibodies. ABD–AaLS may provide new opportunities to develop versatile target-dependent nanoscale theranostic systems.
1. Introduction
For many biotechnological and biomedical applications and in vivo and/or in vitro diagnostics, target specificity and high binding affinity to any given target molecules are essential features. Antibodies have extremely high binding affinity and specificity for their target molecules, and a variety of antibodies against virtually any desired targets can be readily obtained on demand.1–5 Therefore, antibodies have been widely used as ligands for targeted delivery of various therapeutics and/or diagnostics and specific detection of biomarkers in vitro and/or in vivo. As targeting ligands, antibodies are generally chemically conjugated to functional modules, such as delivery vehicles, enzymes, chemicals, or any other supporting materials.6–10 However, chemical conjugation and the related approaches often suffer from decreased affinity and specificity of antibodies generally due to alterations in their target binding sites or destabilization and random orientation of the antibodies.4,11 Therefore, it is necessary to develop a nanoplatform that achieves oriented display of antibodies on the surface without the loss of the essential properties of the antibodies: high affinity and specificity. In addition, a potential nanoplatform should have structural plasticity that acquires additional functionalities on demand as well as a structural uniformity that ensures reproducible outcomes.Lumazine synthase protein cage nanoparticle isolated from the hyperthermophile Aquifex aeolicus (AaLS) is a nanocage-forming enzyme that consists of 60 identical subunits with an exterior diameter of 15.4 nm and an 8 nm interior cavity,12 and catalyzes the penultimate step in the synthesis of riboflavin.13 Its hollow spherical architecture has been used as a template for the encapsulation of cargo proteins, such as green fluorescent protein,14,15 HIV protease,16 and ferritin,17 by engineering the electrostatic properties of its interior surface and the biomineralization of iron oxide nanoparticles.18 AaLS has also been utilized as a building block for fabricating uniform layer-by-layer (LbL) assemblies19 and as a nanoplatform for developing a versatile drug delivery vehicle.20,21 Similar to the other protein cage nanoparticles, such as ferritin, virus-like particles and encapsulin, AaLS has a uniform size distribution and a symmetric and well-defined multivalent structure.12 Furthermore, AaLS exhibits an unusual heat stability and genetic and chemical versatility that allows it to acquire multiple functionalities simultaneously.19–21
In this study, we constructed a universal multivalent antibody-binding nanoplatform by genetically introducing antibody-binding domains (ABDs) to the surface of lumazine synthase protein cage nanoparticles isolated from the hyperthermophile Aquifex aeolicus (AaLS). We demonstrate that the resulting ABD–AaLS effectively captures various types of antibodies derived from diverse species on demand, and that their complexes selectively recognize and bind to their target cells in vitro, guided by antibodies displayed on the surface of ABD–AaLS (Scheme 1).
 | ||
| Scheme 1 Construction of ABD–AaLS protein cage nanoparticle and its application for fluorescent cell imaging as polyvalent antibody-displaying nanoplatforms. | ||
2. Experimental
2.1 Construction and purification of antibody-binding domain displaying AaLS (ABD–AaLS) protein cage nanoparticles
The IPTG inducible pET-30b based plasmids containing genes encoding wild-type AaLS protein was prepared and used as templates. The optimized antibody-binding domain (ABD) was synthesized and subcloned into the C-terminus of AaLS gene with extra linker residues. We introduced 26 amino acid residues to provide sufficient flexibility and space for antibody binding. The amplified DNAs were used to transform the competent E. coli strain BL21 (DE), resulting in the over-expression in E. coli of the ABD–AaLS protein cage nanoparticles.2.2 Quartz crystal microbalance (QCM) measurements
QCM experiments were performed using Q-Sense E4 and standard gold QCM sensors (Q-Sense, Sweden) as described previously, with slight modifications.4 Briefly, the system was operated in flow mode with a pump and temperature was maintained at 25.0 ± 0.1 °C. Each sample solution was introduced to the measurement chamber with a pump and continuously measured for 10 min prior to the subsequent introductions. AaLS and ABD–AaLS and various IgGs (rabbit, rat, and mouse) were introduced at concentrations of approximately 100 μg ml−1 and 50 μg ml−1, respectively, in phosphate buffer (50 mM phosphate, 100 mM NaCl, pH 6.5). Resonance frequencies were measured simultaneously at seven harmonics (5, 15, 25, 35, 45, 55 and 65 MHz). For clarity, only the normalized frequency of the third overtone is shown.2.3 Surface plasmon resonance (SPR) analysis
SPR experiments were performed with carboxyl dextran CM5 gold chips on a Biacore 3000 device (Biacore AB, Sweden) at 25 °C using a PBS buffer as a running solution. Rabbit, rat, or mouse IgGs were coupled to the surface of a CM5 sensor chip by standard amine-coupling chemistry on the SPR instrument as described previously, with slight modifications.4,22 Briefly, a mixture of EDC (0.4 M) and NHS (0.6 M) was injected onto the chip at a flow rate of 10 μl min−1 to activate carboxyl groups on the sensor surface and subsequently 20 μg ml−1 of rabbit, rat, or mouse IgGs were added at the same flow rate for 7 min. Excess reactive groups were blocked with 1 M ethanolamine (pH 8.0). ABD–AaLS captures by rabbit, rat, or mouse IgGs were examined by applying various amounts (30, 60, 120, 240, and 400 nM) of ABD–AaLS (PBS, pH 7.4) to the surface at a flow rate of 30 μl min−1. AaLS was applied in parallel as a control.2.4 Cell culture and fluorescence cell microscopy
SKBR3 was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and SCC-7 and KB cells were obtained from the Korean cell line bank (KCLB). SKBR3 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS, Invitrogen Corp., Carlsbad, CA) and antibiotics (100 μg ml−1 penicillin and 50 μg ml−1 streptomycin) at 37 °C under 5% CO2. SCC-7 cells were cultured in RPMI1640 supplemented with 10% FBS and 1% penicillin–streptomycin and KB cells were cultured in RPMI1640 medium with L-glutamine (300 mg l−1), 10% FBS, 25 mM HEPES and 25 mM NaHCO3. SKBR3, SCC-7, and KB cells (2 × 104 per well) were grown in 8-well microscopy chambers (Ibidi GmbH, Martinsried, Germany).23–25 The combinations of fABD–AaLS (final 50 nM) and Alexa-HER2-Ab or HER2-Ab (rabbit IgG, final 200 nM), fABD–AaLS (final 50 nM) and Alexa-CD44-Ab or CD44-Ab (rat IgG, final 200 nM), or fABD–AaLS (final 50 nM) and Alexa-integrin-Ab or integrin-Ab (mouse IgG, final 200 nM) were mixed in DMEM supplemented with 10% (v/v) FBS, antibiotics, and 0.05% Tween 20, and incubated at room temperature for 30 min. The individual mixtures of fABD–AaLS and various IgGs were added to corresponding cell culture wells and incubated at 37 °C for 30 min. The cells were washed three times with PBS containing 0.05% Tween 20, and fixed with 4% paraformaldehyde at room temperature for 20 min, and again washed with PBS. The cells were incubated with 4,6-diamidino-2-phenylindole (DAPI, Sigma) in PBS for 10 min and washed with PBS. Fluorescence images are obtained using a Personal DV microscope (Applied Precision, Washington, USA).3. Results and discussion
We first synthesized a gene that encodes the ABD of protein A to construct an AaLS-based multivalent antibody-binding nanoplatform. Protein A and G have been widely used as ligands for purifying various types of antibodies, because they selectively capture antibodies with high affinity.11,26 In particular, the ABD of protein A (the so-called Z-domain) is known to exclusively bind to the Fc region of various types of antibodies.27–30 We also genetically engineered AaLS by replacing arginine 108 with cysteine (R108C) for later use in site-specific modification with diagnostic probes such as fluorescent dyes.19–21 Subsequently, we fused the ABD nucleic acid sequence to the 3′ end of the AaLS gene with intervening linker residues (KDPNSGGGLVPRGSGGGSGGGTGGGSGGG) to provide flexibility and room for antibody accessibility. We previously reported that the C-termini of AaLS expose on the surface of protein cage nanoparticles and tolerate the insertion of oligopeptides.19–21 However, this is the first time that a domain of this size (59 amino acids) has been introduced.We overexpressed the ABD–AaLS and successfully purified them without significant loss of materials. We confirmed the fusion of the ABD and AaLS using DNA sequencing and mass spectrometric analysis. Compared with the mass of the dissociated AaLS monomer (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652.5 Da), the dissociated subunit mass of ABD–AaLS (25
652.5 Da), the dissociated subunit mass of ABD–AaLS (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557.0 Da) showed an increase of 8904.5 Da, which exactly corresponds to the combined mass of the ABD and linker (calc. 8904.5 Da) (Fig. 1A). In addition, the ABD fused to the C-terminus of AaLS did not significantly alter its original protein cage architecture. Transmission electron microscopic (TEM) images revealed a cage-like architecture with a diameter of approximately 15 nm, which is almost identical to that of AaLS (Fig. 1B). Since the ABD is relatively small and lacking in electron density, it was not readily apparent in the TEM images. However, ABD–AaLS eluted much earlier than AaLS in size-exclusion chromatography (Fig. 1C), consistent with a larger hydrodynamic diameter due to the addition of ABDs on the surface of AaLS. Dynamic light scattering analysis of ABD–AaLS confirmed a larger hydrodynamic diameter (18.3 nm) than that of AaLS (15.8 nm) (Fig. 1D). These results indicate that ABD–AaLS forms an intact protein cage architecture uniformly displaying ABDs on the surface of AaLS.
557.0 Da) showed an increase of 8904.5 Da, which exactly corresponds to the combined mass of the ABD and linker (calc. 8904.5 Da) (Fig. 1A). In addition, the ABD fused to the C-terminus of AaLS did not significantly alter its original protein cage architecture. Transmission electron microscopic (TEM) images revealed a cage-like architecture with a diameter of approximately 15 nm, which is almost identical to that of AaLS (Fig. 1B). Since the ABD is relatively small and lacking in electron density, it was not readily apparent in the TEM images. However, ABD–AaLS eluted much earlier than AaLS in size-exclusion chromatography (Fig. 1C), consistent with a larger hydrodynamic diameter due to the addition of ABDs on the surface of AaLS. Dynamic light scattering analysis of ABD–AaLS confirmed a larger hydrodynamic diameter (18.3 nm) than that of AaLS (15.8 nm) (Fig. 1D). These results indicate that ABD–AaLS forms an intact protein cage architecture uniformly displaying ABDs on the surface of AaLS.
In order to investigate whether the displayed ABDs on the AaLS effectively capture various types of antibodies, we first monitored interactions between antibodies and ABD–AaLS in real time by quartz crystal microbalance (QCM). Deposition and release of molecules on the QCM sensor induce decreases and increases in resonance frequency (−ΔF), respectively, and these changes are sensitive to the masses of the deposited or released molecules.31 We previously showed that wild type and AaLS variants stably form a uniform monolayer on a gold QCM sensor without any surface modifications.4,19,32,33 ABD–AaLS also strongly bound to the gold QCM sensor to form a uniform monolayer and remained bound even after washing with buffer (Fig. 1E). The resonance frequency of the ABD–AaLS monolayer dramatically decreased upon the addition of rabbit IgG, whereas that of the AaLS monolayer was unchanged (Fig. 1E). Even extensive buffer washing did not remove the initially bound rabbit IgGs (Fig. 1E).
To further investigate the binding affinity between ABD–AaLS and rabbit IgGs, we performed surface plasmon resonance (SPR) analyses. In contrast to QCM studies, we first immobilized rabbit IgGs on the surface of an SPR CM5 sensor chip and then introduced ABD–AaLS at several different concentrations (Fig. 1F). Each experiment was performed after regeneration of the free-IgG surface by base washing of ABD–AaLS bound to the IgG on the chip and subsequent equilibration with the appropriate binding buffer.34 Gradual increases in SPR responses (RU) were observed upon introduction of ABD–AaLS at various concentrations (Fig. 1F), whereas no apparent change was observed upon introduction of AaLS (Fig. S1†). Consistent with previous QCM results, apparent release of bound ABD–AaLS from the immobilized IgGs was not observed even after extensive buffer washing (Fig. 1F). These data suggest that the polyvalent display of ABDs on the surface of AaLS protein cage nanoparticles may allow them to capture IgGs cooperatively while tightly maintaining antibody/ABD–AaLS (Ab/ABD–AaLS) complexes.
To investigate whether Ab/ABD–AaLS complexes can selectively bind their target cells guided by bound antibodies, we prepared target cell lines and selected corresponding monoclonal antibodies that recognize these cells. First, we prepared the SKBR3 breast cancer cell line and chose an anti-HER2 antibody as a model rabbit IgG, since HER2 is known to be overexpressed on the surface of SKBR3 cells and has been extensively utilized as a ligand for targeted delivery and therapy.35,36 We first prepared Alexa Fluor 647-labeled anti-HER2 monoclonal antibodies (Alexa-HER2-Ab) and fluorescein-labeled ABD–AaLS (fABD–AaLS). Mass spectrometric analysis revealed that all the subunits were labeled with one fluorescein (60 fluorescein molecules per ABD–AaLS nanoparticle) (Fig. S2A†). Fluorescein conjugation did not significantly alter their cage architecture (Fig. S2B and C†). Subsequently, we simply mixed Alexa-HER2-Ab and fABD–AaLS to form Alexa-HER2-Ab/fABD–AaLS complexes. These complexes were incubated with SKBR3 cells and visualized and analyzed by fluorescence microscopy and flow cytometry. fABD–AaLS and Alexa-HER2-Ab alone were used as negative and positive controls, respectively. While Alexa-HER2-Ab/fABD–AaLS complexes and Alexa-HER2-Ab bound to SKBR3 cells (Fig. 2A and B), fABD–AaLS alone did not (Fig. 2D). Fluorescence images of Alexa-HER2-Ab (Fig. 2A, red, middle panel) and fABD–AaLS (Fig. 2A, green, left panel) indicated their colocalization, which was confirmed in merged images (Fig. 2A, right panel). Furthermore, when we treated SKBR3 cells with a complex of unlabeled HER2-Ab and fABD–AaLS, the cells were well visualized with HER2-Ab/fABD–AaLS complexes without fluorescent labeling of antibodies (Fig. 2C). Flow cytometry measurements of Alexa-HER2-Ab, Alexa-HER2-Ab/fABD–AaLS complexes, and HER2-Ab/fABD–AaLS complexes revealed virtually identical levels of cell binding, quantitatively confirming their cell-specific binding (Fig. 2E). These data demonstrate that anti-HER2 antibodies on the surface of fABD–AaLS allow these complexes to recognize HER2 expressed on the surface of SKBR3 cells and selectively bind to them. These data also imply that ABD–AaLS serves as a nanoplatform that not only displays targeting antibodies on the surface in an orientation-controlled manner but also acquires fluorescent probes effectively, and that they can be used as target-specific cell imaging probes.
To examine whether ABD–AaLS can serve as a universal antibody-binding nanoplatform for target-selective cell imaging by displaying various types of targeting antibodies (IgGs) on the surface in an orientation-controlled manner, we adapted two other types of antibodies derived from rat and mouse individually. The ability of ABD–AaLS to bind to rat and mouse IgGs was validated by QCM and SPR analyses. QCM and SPR studies were performed under the same conditions as those for rabbit IgG, and the patterns of rat and mouse IgGs binding to the ABD–AaLS monolayer on the QCM sensor were virtually identical to those of rabbit IgG (Fig. S3†). For SPR experiments, rat or mouse IgGs were immobilized on the surface of an SPR CM-5 sensor chip instead of rabbit IgG, followed by application of ABD–AaLS at a series of concentrations. The patterns of SPR responses were also similar to those of rabbit IgG (Fig. S4†), suggesting that rat and mouse IgGs also bind strongly to ABD–AaLS but not to AaLS as rabbit IgG does.
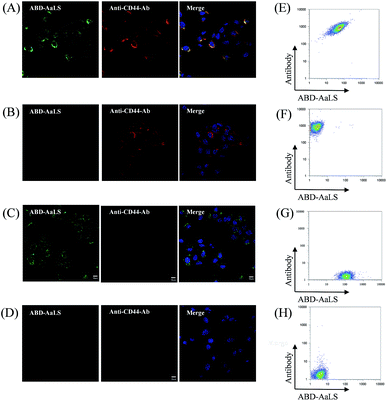
To confirm specific binding between the non-covalent complexes consisting of ABD–AaLS and rat or mouse IgGs to the target cells, we chose anti-CD44 monoclonal antibody and anti-integrin αβγ3 monoclonal antibody as rat- and mouse-derived IgGs, respectively. We performed fluorescence cell imaging and flow cytometry under the same conditions as those for Alexa-HER2-Ab/fABD–AaLS. We prepared Alexa Fluor 647-labeled anti-CD44 Ab (Alexa-CD44-Ab) and anti-integrin αβγ3 Ab (Alexa-integrin-Ab), unlabeled anti-CD44 Ab (CD44-Ab) and anti-integrin αβγ3 Ab (integrin-Ab), and fluorescein-labeled ABD–AaLS (fABD–AaLS), and we subsequently mixed each Ab and fABD–AaLS to form Ab/fABD–AaLS complexes with various combinations as described above. The resulting Alexa-CD44-Ab/fABD–AaLS and CD44-Ab/fABD–AaLS or Alexa-integrin-Ab/fABD–AaLS and integrin-Ab/fABD–AaLS complexes were incubated with SCC-7 cells, which overexpress the cell-surface glycoprotein CD44 that is involved in cell–cell interactions and cell adhesion and migration,37,38 or KB cells, which overexpress integrin αγβ3 on their surface.39 The cells were then examined by fluorescence microscopy and flow cytometry (Fig. 3 and S5†). Similarly to rabbit IgG, Alexa-CD44-Ab/fABD–AaLS and Alexa-integrin-Ab/fABD–AaLS complexes selectively bound to SCC-7 cells and KB cells, respectively (Fig. 3A, E and S5†), and the fluorescence signals of fABD–AaLS (Fig. 3A, green, left panel) and Alexa-CD44-Ab (Fig. 3A, red, right panel) overlapped to each other (Fig. 3A, right panel). Alexa-CD44-Ab and CD44-Ab/fABD–AaLS complexes also tightly bound to SCC-7 cells (Fig. 3B, C, and E). ABD–AaLS was alternatively labeled with Alexa Fluor 647 and subsequently complexed with unlabeled CD44-Ab. These complexes also tightly bound to SCC-7 cells (Fig. S6†), suggesting that ABD–AaLS is amenable to chemical modifications with various types of fluorophores.
To examine the efficacy of antibody-guided targeted drug delivery with ABD–AaLS, the 6-maleimidocaproyl hydrazone prodrug of doxorubicin (AlDox)40 was chemically conjugated to ABD–AaLS (ABD–AaLS–AlDox) instead of fluorophores as described previously.20,25 Subsequently, targeting antibodies were non-covalently complexed with ABD–AaLS–AlDox (Ab/ABD–AaLS–AlDox) depending on target cells and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assays were performed. Hydrazine linkage is known to be quite stable at neutral pH and to become quickly cleaved under an acidic condition (pH 4.5–5.5).40 We previously showed that 48 AlDox molecules are conjugated to one AaLS (60 subunits) and approximately 65% of them are released within 15 h.20
The SKBR3, SCC-7 and KB cells were treated with the complexes of anti-HER2-Ab, anti-CD44-Ab, or anti-integrin-Ab and ABD–AaLS–AlDox, respectively, for 1 h. The free prodrug, AlDox, and ABD–AaLS only were also treated in parallel to serve as positive and negative controls. One hour later, cells were washed with fresh media to remove unbound complexes and free AlDox, further cultured for 48 h, and their viability was measured to investigate the cytotoxicity of delivered drugs. The cytotoxic effects of delivered AlDox on antibody-corresponding target cells significantly increased in a dose-dependent manner and were higher than even those of free AlDox, whereas ABD–AaLS without AlDox had no significant effect on cell viability (Fig. 4). The enhanced cytotoxicity of antibody-mediated ABD–AaLS–AlDox may be due to receptor-mediated endocytosis of whole complexes upon specific binding, which transports increased amounts of AlDox into the target cells, and subsequent low pH-induced release of Dox into the cells.
Taken together, these results indicate that ABD–AaLS can serve as a universal IgG-binding nanoplatform for efficiently displaying IgGs derived from a variety of species, such as rabbit, rat, and mouse, on demand by simple molecular recognition, and those complexes can be used as not only effective optical probes for target-specific cell imaging but also drug-carriers for target-specific treatment.
4. Conclusions
In this study, we constructed a polyvalent antibody-binding nanoplatform, ABD–AaLS, and demonstrated that ABD–AaLS effectively captures various types of antibodies derived from diverse species on demand. We also successfully demonstrated that targeting antibody/ABD–AaLS complexes selectively recognize and bind to their target cells in vitro, guided by antibodies displayed on the surface of ABD–AaLS.Antibodies offer an almost unlimited range of specific targeting moieties, making them attractive targeting ligands for in vitro and/or in vivo diagnostics.1,2,5,41 Depending on the target cells, appropriate antibodies can be selected and simply mixed with ABD–AaLS, which can be independently pre-modified depending on the purposes, to form diagnostic probe complexes. Utilizing ABD–AaLS as a universal antibody-binding nanoplatform, we can overcome the drawbacks associated with chemical conjugation of antibodies through non-covalent complexation as well as introduce additional functionalities to ABD–AaLS without altering antibody activity. Since ABD–AaLS has an additional internal cavity and exterior sites for encapsulation and attachment of cargo molecules such as drugs and diagnostic probes, ABD–AaLS may provide new opportunities to develop versatile target-dependent theranostic delivery systems.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2013R1A1A1008228 & NRF-2010-0028684) and the year of 2015 research fund (1.150095.01) of UNIST (Ulsan National Institute of Science & Technology).Notes and references
- T. M. Allen, Nat. Rev. Cancer, 2002, 2, 750–763 CrossRef CAS PubMed.
- P. Carter, Nat. Rev. Cancer, 2001, 1, 118–129 CrossRef CAS PubMed.
- J. F. Hainfeld, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 11064–11068 CrossRef CAS.
- H. J. Kang, Y. J. Kang, Y.-M. Lee, H.-H. Shin, S. J. Chung and S. Kang, Biomaterials, 2012, 33, 5423–5430 CrossRef CAS PubMed.
- M. von Mehren, G. P. Adams and L. M. Weiner, Annu. Rev. Med., 2003, 54, 343–369 CrossRef CAS PubMed.
- N. Erathodiyil and J. Y. Ying, Acc. Chem. Res., 2011, 44, 925–935 CrossRef CAS PubMed.
- O. C. Farokhzad and R. Langer, ACS Nano, 2009, 3, 16–20 CrossRef CAS PubMed.
- E. R. Gillies and J. M. J. Frechet, Drug Discovery Today, 2005, 10, 35–43 CrossRef CAS PubMed.
- D. Sutton, N. Nasongkla, E. Blanco and J. M. Gao, Pharm. Res., 2007, 24, 1029–1046 CrossRef CAS PubMed.
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145–160 CrossRef CAS PubMed.
- R. Danczyk, B. Krieder, A. North, T. Webster, H. HogenEsch and A. Rundell, Biotechnol. Bioeng., 2003, 84, 215–223 CrossRef CAS PubMed.
- X. Zhang, W. Meining, M. Fischer, A. Bacher and R. Ladenstein, J. Mol. Biol., 2001, 306, 1099–1114 CrossRef CAS PubMed.
- X. Zhang, W. Meining, M. Cushman, I. Haase, M. Fischer, A. Bacher and R. Ladenstein, J. Mol. Biol., 2003, 328, 167–182 CrossRef CAS PubMed.
- F. P. Seebeck, K. J. Woycechowsky, W. Zhuang, J. P. Rabe and D. Hilvert, J. Am. Chem. Soc., 2006, 128, 4516–4517 CrossRef CAS PubMed.
- B. Wörsdörfer, Z. Pianowski and D. Hilvert, J. Am. Chem. Soc., 2012, 134, 909–911 CrossRef PubMed.
- B. Wörsdörfer, K. J. Woycechowsky and D. Hilvert, Science, 2011, 331, 589–592 CrossRef PubMed.
- T. Beck, S. Tetter, M. Künzle and D. Hilvert, Angew. Chem., Int. Ed., 2015, 54, 937–940 CrossRef CAS PubMed.
- W. Shenton, S. Mann, H. Cölfen, A. Bacher and M. Fischer, Angew. Chem., Int. Ed., 2001, 40, 442–445 CrossRef CAS.
- H. Moon, W. G. Kim, S. Lim, Y. J. Kang, H.-H. Shin, H. Ko, S. Y. Hong and S. Kang, J. Mater. Chem. B, 2013, 1, 4504–4510 RSC.
- J. Min, S. Kim, J. Lee and S. Kang, RSC Adv., 2014, 4, 48596–48600 RSC.
- J.-S. Ra, H.-H. Shin, S. Kang and Y. Do, Clin. Exp. Vaccine Res., 2014, 3, 227–234 CrossRef CAS PubMed.
- Y. J. Kang, H. J. Yang, S. Jeon, Y.-S. Kang, Y. Do, S. Y. Hong and S. Kang, Macromol. Biosci., 2014, 14, 619–625 CrossRef CAS PubMed.
- C. Kwon, Y. J. Kang, S. Jeon, S. Jung, S. Y. Hong and S. Kang, Macromol. Biosci., 2012, 12, 1452–1458 CrossRef CAS PubMed.
- J. Min, H. Moon, H. J. Yang, H.-H. Shin, S. Y. Hong and S. Kang, Macromol. Biosci., 2014, 14, 557–564 CrossRef CAS PubMed.
- H. Moon, J. Lee, J. Min and S. Kang, Biomacromolecules, 2014, 15, 3794–3801 CrossRef CAS PubMed.
- K. Bonroy, F. Frederix, G. Reekmans, E. Dewolf, R. De Palma, G. Borghs, P. Declerck and B. Goddeeris, J. Immunol. Methods, 2006, 312, 167–181 CrossRef CAS PubMed.
- A. C. Braisted and J. A. Wells, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 5688–5692 CrossRef CAS.
- J. Deisenhofer, Biochemistry, 1981, 20, 2361–2370 CrossRef CAS PubMed.
- J. Lee, E. K. Song, Y. Bae, J. Min, H.-W. Rhee, T. J. Park, M. Kim and S. Kang, Chem. Commun., 2015, 51, 10945–10948 RSC.
- M. P. Hwang, J.-W. Lee, K. E. Lee and K. H. Lee, ACS Nano, 2013, 7, 8167–8174 CrossRef CAS PubMed.
- F. Höök, M. Rodahl, P. Brzezinski and B. Kasemo, Langmuir, 1998, 14, 729–734 CrossRef.
- S. Kang, P. A. Suci, C. C. Broomell, K. Iwahori, M. Kobayashi, I. Yamashita, M. Young and T. Douglas, Nano Lett., 2009, 9, 2360–2366 CrossRef CAS PubMed.
- Y. J. Kang, M. Uchida, H. H. Shin, T. Douglas and S. Kang, Soft Matter, 2011, 7, 11078–11081 RSC.
- Y. W. Jung, H. J. Kang, J. M. Lee, S. O. Jung, W. S. Yun, S. J. Chung and B. H. Chung, Anal. Biochem., 2008, 374, 99–105 CrossRef CAS PubMed.
- I. Hellstrom, G. Goodman, J. Pullman, Y. Yang and K. E. Hellstrom, Cancer Res., 2001, 61, 2420–2423 CAS.
- A. Rhodes, Cancer Biomarkers, 2005, 1, 229–232 CAS.
- K. Y. Choi, H. Chung, K. H. Min, H. Y. Yoon, K. Kim, J. H. Park, I. C. Kwon and S. Y. Jeong, Biomaterials, 2010, 31, 106–114 CrossRef CAS PubMed.
- H. Koo, M. S. Huh, I.-C. Sun, S. H. Yuk, K. Choi, K. Kim and I. C. Kwon, Acc. Chem. Res., 2011, 44, 1018–1028 CrossRef CAS PubMed.
- W. Arap, R. Pasqualini and E. Ruoslahti, Science, 1998, 279, 377–380 CrossRef CAS PubMed.
- D. Willner, P. A. Trail, S. J. Hofstead, H. D. King, S. J. Lasch, G. R. Braslawsky, R. S. Greenfield, T. Kaneko and R. A. Firestone, Bioconjugate Chem., 1993, 4, 521–527 CrossRef CAS PubMed.
- P. A. Suci, S. Kang, M. Young and T. Douglas, J. Am. Chem. Soc., 2009, 131, 9164–9165 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional supplementary data. See DOI: 10.1039/c6ra00233a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |