Superhydrophobic and H2S gas sensing properties of CuO nanostructured thin films through a successive ionic layered adsorption reaction process
Abstract
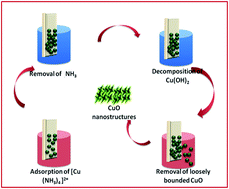
Superhydrophobic surfaces of CuO were synthesized using a successive ionic-layered adsorption reaction technique by varying the number of deposition cycles followed by a thermal annealing process. The prepared superhydrophobic surface was composed of monoclinic CuO, which was confirmed by X-ray diffraction and X-ray photoelectron spectroscopy. Dynamic contact angle measurements were obtained using a droplet evaporation method. The contact angles measured on CuO surfaces after 20, 40, and 60 deposition cycles were similar, all showing hydrophilicity. After the thermal treatment, the prepared films exhibit super-hydrophobicity with a high water contact angle of approximately 157°, which contributed to the stable superhydrophobic surface. A considerable increase in surface roughness (from 23.81 to 74.54 nm) and the conical-shaped CuO nanostructures led to super-hydrophobicity. Also a possible mechanism for the wettability behavior is proposed. Finally, the superhydrophobic CuO surface which was used for the detection of H2S gas showed a very fast response. These results indicate that the low temperature deposition of CuO nanostructured thin films were promising for reliable high-performance gas sensors.

 Please wait while we load your content...
Please wait while we load your content...