Spectrofluorimetric determination of Hg2+ and Pb2+ using acetylcholinesterase (AChE)-based formation of silver nanoparticles†
D. Nanda Kumar,
Jaydeep Roy,
S. A. Alex,
N. Chandrasekaran and
A. Mukherjee*
Centre for Nanobiotechnology, VIT University, Vellore – 632014, India. E-mail: amit.mookerjea@gmail.com; amitav@vit.ac.in; Fax: +91 416 2243092; Tel: +91 416 2202620
First published on 15th February 2016
Abstract
We herein report a novel fluorimetric detection method for Hg2+ and Pb2+ based on in situ formation of AgNPs, where thiocholine (TCh), a product obtained by the hydrolysis of acetylcholine (ACh) by acetylcholinesterase (AChE), can act as a stabilizing agent. Uniformly dispersed AgNPs were obtained in the control sample (in the presence of optimized concentrations of AChE, ATCh, AgNO3 and NaBH4) without addition of any inhibitors (Hg2+ or Pb2+), resulting in decreased fluorescence intensity. However, the addition of Hg2+ and Pb2+ to the reaction mixture tends to decrease the vibrational and rotational speed with an increase in the aggregate diameter of particles, and thus increases the emission intensity of AgNPs at 423 nm. The increase in the emission intensity can be well correlated to the increase in concentration of metal ions. The limit of detection (LOD) was found to be 1.47 × 10−14 M for Hg2+ and 0.324 × 10−10 M for Pb2+, and the proposed method was successfully used for the determination of Hg2+ and Pb2+ in environmental samples. Further, this sensor model was also employed to perform the concurrent detection of Hg2+ in the presence of Pb2+ or vice versa in aqueous solutions.
1. Introduction
The prominent discharge of metal-contaminated wastewater to the environment by industrial effluents causes serious health effects. The major impact of heavy metals is on soils, plants, and human beings. The microbial and enzymatic activities of microorganisms in the soils are significantly inhibited by heavy metals due to soil contamination,1 and the cellular uptake of various heavy metal contaminates by plants exceeds their tolerance and can cause plants to die.2 When human beings are exposed over a long time to low levels of heavy metals through food, water, air and commercial products, it causes several health problems. Heavy metal toxicity may cause damage to the central nervous system (CNS), cardiovascular system, gastrointestinal system, organs, and reduced growth and development.3 These heavy metals indirectly inhibit the active site of acetylcholinesterase (AChE), which breaks down acetylcholine (ACh) at cholinergic synapses.4Heavy metals like mercury (Hg), cadmium (Cd), lead (Pb), chromium (Cr), arsenic (As) etc., are widely used in many industries as commercial products.2 Mercury, which exists in metallic, organic, and inorganic forms, has attracted a lot of attention due to its high toxicity. Hg2+ is more stable in the form of inorganic mercury due to its high solubility in aqueous solutions.5 Elemental mercury is widely used in fluorescent lamps, batteries, mercury vapor lamps, electrical switches, thermometers, rectifiers, electrodes, and in medical fields as dental fillings, which are the major sources of Hg2+ discharge into the environment.6 Similarly, lead (Pb) also exists in different forms in natural sources. Pb2+ is found to exhibit acute toxicity in human beings, and a long-term exposure of Pb2+ can have harmful effects on the biological system, and it does not undergo biodegradation.7 The major sources of Pb2+ discharge are batteries, paint and fertilizer industries, and petrol additives.8 According to USEPA, the permissible limits for Hg and Pb ions in drinking water are 10 × 10−9 M and 72 × 10−9 M, respectively.
Though heavy metals are useful for various applications, they are considered as high density chemical components, and hence, are toxic even at low concentrations. Although varying amounts of some heavy metals like iron (Fe), zinc (Zn), copper (Cu), manganese (Mn), and cobalt (Co) are required by living organisms in lower quantities, even these metals are toxic at higher concentrations.9 Among all the heavy metals, mercury and lead are considered hazardous due to their ability to show high toxicity even at lower concentrations. Thus, the determination of heavy metals like Hg2+ and Pb2+ in environmental samples with high sensitivity and selectivity, without any interference from other metal ions, is essential.
There are some techniques like cold-vapor atomic absorption spectroscopy (CVAA),10 cold-vapor atomic fluorescence spectroscopy (CVAF),11 inductively coupled plasma mass spectrometry (ICP-MS),12 voltammetry,13 colorimetry,14 and spectrophotometry,15 which have been used to measure heavy metals, especially Hg2+ and Pb2+. Though many of these techniques are modern and have advantages to detect different quantities of heavy metals from any sample matrix, they also have some disadvantages as they are time consuming, consume lots of energy, have low sensitivity and selectivity, and require high-cost and advanced instruments.16 Nowadays, nanomaterials in the field of biosensors attract more attention towards the determination of heavy metals due to their high sensitivity in chemical and biological sensing.17
To date, various sensor methods that have been used to determine Hg2+ based on organic compounds,18,19 oligonucleotides,20 conjugated polymers,21,22 DNAzyme,23,24 nanoparticles,25,26 and nanorods,27,28 have been reported. Similarly, different spectrophotometric methods have been reported for the detection of Pb2+ (ref. 14, 17, 29 and 30) using DNAzyme-directed assembly of AuNPs31,32 and other modified NPs,33 peptides,34 and proteins.35 Though earlier reported methods could successfully determine Hg2+ and Pb2+, NP modification and the complicated process for NP preparation, time-consuming sample preparation and preconcentration procedures limit their applications. In addition, these methods could not achieve the detection of Hg2+ and Pb2+ at trace levels with very high sensitivity and selectivity.
However, some of these methods could achieve Hg2+ and Pb2+ detection with high selectivity and sensitivity. Recently, Li et al. demonstrated the detection of Hg2+ and cysteine (Cys) using graphene quantum dots (GQDs) as a fluorescent probe. The GQDs can get quenched by fluorescence charge transfer when Hg2+ is added to the system, and their fluorescence was enhanced and restored when Cys combined with Hg2+ (due to a strong metal–thiol bond formation). The limit of detection (LOD) for Hg2+ and Cys were 0.439 × 10−9 M and 4.5 × 10−9 M, respectively. However, the preparation of GQDs is quite complicated and time consuming.36 Similarly, Lee et al. reported the use of rhodamine 6G (R6G) and 3-mercaptopropionic acid (MPA)-modified AuNPs (R6G/MPA-AuNPs) for sensing Hg2+, and bovine serum albumin (BSA)-capped AuNPs in the presence of thiosulfate (S2O32−) and 2-mercaptoethanol (2-ME) for the detection of Pb2+ in highly saline media. These processes are complicated and require more time to prepare and modify the NPs. The systems could attain an LOD of ca. 2.0 × 10−9 M for Hg2+ and ca. 5.0 × 10−9 M for Pb2+.29 Guo et al. developed a rapid colorimetric detection technique for Hg2+, Pb2+ and Cu2+ using papain-functionalized gold nanoparticles (P-AuNPs). The developed system can detect Hg2+, Pb2+ or Cu2+ as low as 200 × 10−9 M using P-AuNPs, but it is very time consuming as it requires about 24 h to functionalize the papain on AuNPs.14
The detection of Hg2+ and Pb2+ using these existing methods mainly requires fluorophores, organic dyes, or functionalized NPs to improve the selectivity and sensitivity of the system. Recently, in our previous work we reported the sensing of organophosphorus pesticides like trichlorfon and malathion in aqueous solution by an enzyme-based method using the modulated synthesis of AgNPs that can be prepared using sodium citrate as a capping and stabilizing agent.37 In the current work, we describe a novel approach to detect Hg2+ and Pb2+ using fluorescence spectroscopy by thiol-induced formation of AgNPs through an enzyme-based method. In the present work, there is no external stabilizing or capping agent added, and a mild reducing agent is sufficient to form nanoparticles using an enzymatic reaction. The principle of the system is mainly based on the formation of stable AgNPs by the enzymatic generation of thiol (–SH) groups, which act as a stabilizing agent and can also control the particle size of AgNPs. Hg2+ and Pb2+ were found to highly inhibit the enzyme activity among all the other heavy metals. The addition of these heavy metals (Hg2+ and Pb2+) to the system can destabilize the NPs by hindering the enzyme activity, which can cause an increase in the fluorescence emission intensity of AgNPs at 423 nm.
To the best of our knowledge the present work demonstrated for the first time that the fluorimetric detection of Hg2+ and Pb2+ using thiocholine-mediated stabilization can be achieved by an enzymatic reaction during the synthesis of AgNPs. This method was able to achieve the lowest detection level so far for Hg2+ (1.47 × 10−14 M) and Pb2+ (0.324 × 10−10 M) compared with earlier detection methods that have employed fluorescent carbon nanoparticles, silver and gold clusters, carbon nanodots, AuNPs and DNA (Table 1 and 2). The detection of Hg2+ was performed during the synthesis of AgNPs, unlike the other studies reported, which may have resulted in the enhanced sensitivity. The use of biological recognition molecules (enzymes) having high specificity towards interactions with the heavy metals, also played a major role in ultrasensitive detection. The system was also able to concurrently determine the concentrations of both Hg2+ and Pb2+ present in aqueous solution. The application of the developed method was successful for the determination of Hg2+ and Pb2+ in tap and lake water samples.
| Reagent | LODa (M) | Ref. |
|---|---|---|
| a Limit of detection.b System included AgNO3, NaBH4, AChE, and ATCh. | ||
| Au nanoclusters | 0.5 × 10−9 | 47 |
| Structure-switching DNA | 3.2 × 10−9 | 48 |
| Ag clusters | 10 × 10−9 | 49 |
| Carbon NPs | 0.23 × 10−9 | 50 |
| Carbon nanodots | 4.2 × 10−9 | 51 |
| Thrombin-binding aptamer (TBA) probe | 5.0 × 10−9 | 52 |
| During synthesis of AgNPsb | 1.47 × 10−14 | Present work |
| Reagent | LODa (M) | Ref. |
|---|---|---|
| a Limit of detection.b Cadmium tellurium quantum dots.c 2-Mercaptoethanol (2-ME)–thiosulfate (S2O32−)–AuNPs.d System included AgNO3, NaBH4, AChE, and ATC. | ||
| DNAzyme and AuNPs | 3.0 × 10−9 | 32 |
| Gallic acid-capped AuNPs | 10.0 × 10−9 | 33 |
| Thrombin-binding aptamer (TBA) probe | 0.3 × 10−9 | 52 |
| CdTe QDsb | 2.7 × 10−7 | 53 |
| 2-ME/S2O32−–AuNPsc | 0.5 × 10−9 | 54 |
| During synthesis of AgNPsd | 0.32 × 10−10 | Present work |
2. Experimental
2.1. Materials
Acetylthiocholine iodide (ATChI), acetylcholinesterase (from Electrophorus electricus), and mercury(II) chloride (Hg2+) were obtained from Sigma-Aldrich, India. Silver nitrate (AgNO3) and sodium borohydride (NaBH4) were purchased from SRL Pvt. Ltd (India). Tris(hydroxymethyl) aminomethane and the heavy metal salts Mn2+, Fe2+, Fe3+, Pb2+, and Cr6+ were obtained from SRL Pvt. Ltd (India), Ni2+, Cr3+, Zn2+, Cd2+, and Mg2+ were from Sigma-Aldrich, India.The stock solutions for all the heavy metals (1 mM) were prepared freshly in Milli-Q water, and the heavy metals were further diluted to the appropriate concentration for further experimental use and spectroscopic studies. An AChE stock solution (100 mU mL−1) was made by using tris buffer (10 mM) and further, required concentrations were diluted with tris buffer and stored at 4 °C when not in use. The ATChI stock solution (10 mM) was also prepared using tris buffer (10 mM). The iodide ions in the ATChI solution were removed by our previous method to enhance the limit of detection.38 Analytical grade chemical reagents were used throughout the experiments without further purification, and all the solutions were prepared using Milli-Q water unless stated otherwise.
2.2. Instrumentation
UV absorption spectra were recorded on a UV-2600 spectrophotometer (Shimadzu, Tokyo, Japan). All the measurements were made in the spectral range from 200 to 800 nm. Fluorescence emission spectra were obtained using a Cary eclipse fluorescence spectrophotometer (Agilent Technologies, Model-G9800A). The emission wavelength at 423 nm corresponding to the excitation at 340 nm was recorded, and the spectral wavelength range studied was from 400 to 450 nm. Additional tests were carried out to confirm that the obtained emission peak of AgNPs at 423 nm was not due to a Rayleigh scattering phenomenon by varying the excitation value from 300 to 380 nm, and the excitation peak of AgNPs was constantly obtained at 340 nm (data not shown). The surface morphological studies of AgNPs and the particle size of the dispersed and aggregated AgNPs were obtained using transmission electron microscopy (TEM, FEI Company Tecnai™, G2 20 Twin) at an accelerating voltage of around 200 kV. The particle size distribution of the AgNPs before and after addition of Hg2+ and Pb2+ in the presence of AChE and ATCh was obtained using dynamic light scattering (DLS) analysis (NanoBrook 90Plus PALS Particle Size Analyzer, Brookhaven Instruments Corporation, USA).2.3. Detection of Hg2+
The fluorimetric determination of Hg2+ concentration was measured by studying the spectra of AgNPs with an excitation wavelength of 340 nm, and their emission spectra were recorded from 400 to 450 nm. A maximum emission intensity was obtained at 423 nm, which corresponds to the SPR band of AgNPs formed during the synthesis process. Initially, the formation of AgNPs (control sample without addition of Hg2+) was followed by the addition of 40 μL of AChE (4 mU), 80 μL of ATCh (1 mM), 400 μL of AgNO3 (0.1 mM) and 80 μL of NaBH4 (1 mM) respectively, and the reaction mixtures were diluted up to 800 μL with Milli-Q water. A pale yellow color was observed during the formation of NPs (details are provided in ESI Fig. S1A†). Subsequently, 50 μL of each concentration of Hg2+ from 1 to 100 × 10−13 M was added to the reaction mixture in each vial. Then, the reaction mixture of each vial was incubated for 15 min at 37 ± 2 °C. After incubation, the color of the reaction mixture changed from pale yellow to grey (details are provided in ESI Fig. S1B†), and concomitantly the increase in fluorescence emission intensity was recorded at 423 nm for each sample. As the concentration of Hg2+ increases, an increase in the size of NPs occurs, and the reaction mixture without Hg2+ addition indicated the formation of stable NPs in the presence of AChE and ATCh. All the experiments were performed in triplicate to acquire reproducible data.2.4. Detection of Pb2+
The different concentrations of Pb2+ from 0.1 to 10 × 10−9 M (50 μL each concentration) were incubated with the reaction mixtures, which contained 40 μL of AChE (4 mU), 80 μL of ATCh (1 mM), 400 μL of AgNO3 (0.1 mM) and 80 μL of NaBH4 (1 mM) respectively, and the final volume of the solution was adjusted to 800 μL with Milli-Q water. Then, the reaction mixture of each concentration of Pb2+ was incubated for 20 min at 37 ± 2 °C. The florescence spectrum of each solution was recorded immediately at 423 nm, and then the measured spectral changes in the emission intensity were used to plot the calibration curve for Pb2+. In this system, the duration of incubation time was observed to be higher because the binding capacity and degree of inhibition efficiency of Pb2+ is much lower compared with Hg2+. After incubation, the color of the solution changed from pale yellow to grey when Pb2+ was added during the formation of AgNPs.2.5. Concurrent detection of both Hg2+ and Pb2+
The detection of both heavy metal ions, Hg2+ and Pb2+, was ascertained by maintaining a fixed concentration of one metal and their spectral changes were studied by fluorescence spectroscopy. Different concentrations of Hg2+ (1 to 100 × 10−13 M) were added in the presence of a fixed concentration of Pb2+ (2 × 10−9 M), and 50 μL of both metal ion mixtures was added to the system containing 40 μL of AChE (4 mU), 80 μL of ATCh (1 mM), 400 μL of AgNO3 (0.1 mM), and 80 μL of NaBH4 (1 mM). The reaction volume was adjusted to 800 μL with Milli-Q water. Then, the reaction mixture was incubated for 20 min at 37 ± 2 °C. The spectral changes in emission intensity at 423 nm was recorded immediately after the reaction was complete. Similarly, when changing the concentration of Pb2+ (0.1 to 10 × 10−9 M), maintaining a constant concentration of Hg2+ (1 × 10−13 M) and incubating the resulting mixtures for 20 min, a significant increase in the emission intensity at 423 nm was noticed, and the obtained spectral measurements were used to plot the linear calibration graph for Pb2+ in the presence of Hg2+.2.6. Real sample analysis
The practical application of the developed method was estimated by determining the Hg2+ and Pb2+ in real samples. The real samples used were tap and lake water, wherein the lake water samples were collected from the Vellore Institute of Technology Lake (VIT University, Vellore, India) and the tap water samples were collected in our laboratory. The collected lake water was filtered through a 0.22 μm membrane filter to remove foreign residue materials. The pH of both tap (pH 7.1 ± 0.1) and lake (pH 6.9 ± 0.1) water was recorded before spiking the metal ions, and there was no significant change in the pH even after completion of the analysis. The stock solutions were prepared by spiking higher concentrations of both metal ions separately. From the stock solutions, three different working solutions were prepared for Hg2+ (5, 10, and 60 × 10−13 M) and Pb2+ (0.5, 1, and 5 × 10−9 M), respectively. The control sample was also prepared using the same filtration procedure, but it did not contain either metal ions. When the real samples alone were added to the system (in the absence of both metal ions), they did not cause any increase in the fluorescence intensity.3. Results and discussion
3.1. Developing a sensor model for the detection of heavy metals (Hg2+ and Pb2+) using in situ enzymatic formation of AgNPs
We have developed a sensor model for the detection of Hg2+ and Pb2+ using AgNPs, wherein thiocholine (TCh) (a product that is obtained by the hydrolysis of ATCh using AChE) can act as a stabilizer during the formation of stable AgNPs.Initially, the formation of AgNPs involves the optimization of AgNO3 and NaBH4 concentrations, and this was studied by using fluorescence spectroscopy. The emission intensity of AgNPs was recorded at 423 nm with an excitation wavelength at 340 nm. The changes in fluorescence signal with different concentrations of AgNO3 and NaBH4 have been studied by varying the concentration of AgNO3 (0.1–0.001 mM) and fixing the concentration of NaBH4 (1 mM) without the addition of AChE and ATCh. As shown in Fig. 1A, the emission intensity of AgNPs at 423 nm decreased as the concentration of AgNO3 increased while using a fixed concentration of NaBH4 (1 mM). Similarly, as the concentration of NaBH4 (1–0.01 mM) increases the emission intensity decreased slightly with a fixed concentration of AgNO3 (0.1 mM), as shown in Fig. 1B. However, at a fixed concentrations of AgNO3 (0.1 mM) and NaBH4 (1 mM), there is slight decrease in emission intensity at 423 nM as shown in Fig. 1A and B (as compared to other concentrations of AgNO3 and NaBH4).
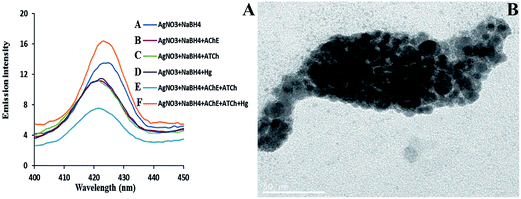
On the addition of AgNO3 and NaBH4, the as-formed AgNPs were found to aggregate immediately and thus formed a less stable colloidal solution owing to the absence of a stabilizing agent. This was further confirmed by transmission electron microscopy (TEM); Fig. 2A shows the AgNPs (0.1 mM of AgNO3, 1 mM of NaBH4), but the particles are unstable and accumulate due to a lack of stabilizing agent. The interaction of AChE and ATCh produces a trace amount of TCh, which can act as a stabilizing agent during the formation of AgNPs. Therefore, the formation of more stable AgNPs was achieved when AChE, ATCh (under the optimized conditions of AChE 4 mU, and ATCh 1 mM), AgNO3 and NaBH4 are all used. Fig. 1A and B show a sudden decrease in the emission intensity of AgNPs at 423 nm in the presence of AChE, ATCh, AgNO3 and NaBH4 (control). The decrease in emission intensity was attributed to the increased stability of AgNPs in the presence of an enzyme–substrate complex compared with using AgNO3 and NaBH4 alone, and the color of the solution was found to change to pale yellow. Further, the addition of AChE and ATCh to the system produces more stable AgNPs with controlled particle size. The formation of AgNPs was corroborated by TEM. Fig. 2B suggests that the formation of AgNPs was stabilized by trace amounts of a thiol-bearing compound, TCh, and their average particle diameter was found to be 9 ± 1 nm.
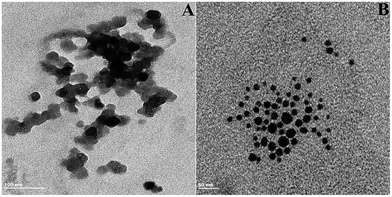 | ||
| Fig. 2 TEM images for AgNPs formed in the presence of (A) AgNO3 (0.1 mM) and NaBH4 (1 mM) and (B) AgNO3 (0.1 mM), NaBH4 (1 mM), AChE (4 mU), and ATCh (1 mM). | ||
The particle size distribution of AgNPs before and after addition of AChE and ATCh with Hg2+ and Pb2+ was further confirmed using dynamic light scattering (DLS) analysis. The mean hydrodynamic size of AgNPs (AgNO3 and NaBH4) was found to be around 100 ± 2 nm (for details see ESI Fig. S2A†). The addition of AChE and ATCh to AgNO3 and NaBH4 during the formation of AgNPs caused the particle size of AgNPs to decrease to 66 ± 1 nm (for details see ESI Fig. S2B†). The diameter of AgNPs in the presence of AChE and ATCh is observed to be larger than the core diameter measured by TEM (9 ± 1 nm), because during DLS measurement, the forward angle is more heavily biased towards the aggregated molecules than the smaller molecules, and thus, the analysis captures the hydrodynamic diameter.39,40 The addition of the heavy metal, either Hg2+ (10 × 10−13 M) or Pb2+ (1 × 10−9 M), to the system during the synthesis of AgNPs results in the accumulation of AgNPs, and the particle size of AgNPs was found to increase to 125 ± 1 nm for Hg2+ and 120 ± 0.5 nm for Pb2+ (for details see ESI Fig. S2C and S2D†), respectively.
The formation of AgNPs was further confirmed by UV-visible spectroscopy using the absorption spectral changes during the addition of AgNO3 and NaBH4. Similarly, experiments were repeated to investigate the changes in absorption intensity of AgNPs with different concentrations of AgNO3 and NaBH4. The absorption peak intensity at 420 nm increased slightly as the concentration of AgNO3 (0.1–0.001 mM) increased by keeping the concentration of NaBH4 fixed (1 mM) Fig. S3A (ESI†). Similarly, as the concentration of NaBH4 (1–0.01 mM) increases, the intensity at 420 nm increased slightly when a fixed concentration of AgNO3 (0.1 mM) was used, as shown in Fig. S3B.† In the control sample, a sudden increase in the intensity of AgNPs was observed at 420 nm as shown in both Fig. S3A and S3B (ESI†). The increase in intensity can again be attributed to the stability of AgNPs in the presence of the enzyme–substrate complex when compared with the usage of AgNO3 and NaBH4 alone. The details of all these experiments are provided in the ESI.†
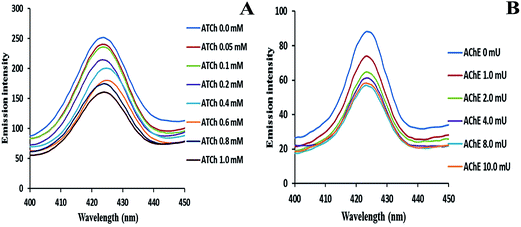
To find out the minimum concentration of AChE and ATCh required to produce stable AgNPs another series of tests were carried out. The fluorescence response was investigated by using different concentrations of ATCh and fixing the amount of AChE. As the concentration of ATCh increases (0 to 1 mM), the response of the fluorescence signal decreases, and the system showed maximum stability in the formation of AgNPs at 1 mM of ATCh with 4 mU of AChE, as shown in Fig. 3A. As the concentration of ATCh increased, the stability of the AgNPs increased, and the system reached saturation after 1 mM of ATCh. In the same manner, the effect of AChE concentration was studied by fixing the amount of ATCh (1 mM). Similarly, as the concentration of AChE increases (0 to 10 mU), there was a gradual decrease in fluorescence as shown in Fig. 3B. Maximum stability of AgNPs was achieved when the AChE concentration was 4 mU. A further increase in the concentration of AChE above 4 mU, resulted in the saturation of the system. For the rest of the experiments, the concentrations of AChE (4 mU) and ATCh (1 mM) were fixed in order to obtain more stable AgNPs.
Heavy metals can act as indirect inhibitors for acetylcholinesterase and can reduce the enzyme activity by inhibiting the active sites of AChE. In this work, the enzyme–substrate complex formation was found to yield more stable AgNPs, and the addition of different concentrations of heavy metals (Hg2+ or Pb2+) to this system could cause an increase in the size of AgNPs and a decrease in the stability of NPs gradually as the concentration of heavy metal was increased. Based on this concept, a sensor model can be developed for the indirect determination of heavy metals using thiol-stabilized AgNPs.
3.2. Mechanism for fluorescent enhancement
The observed increase in emission intensity can be due to the reduction in the vibrational and rotational speed with increasing aggregate diameter of particles, and this is indicated by the decrease in the rate of nonradiative decay.41 The instability in Brownian movement and decrease in colloidal probability between the AgNPs results in the increase in external energy transfer and quantum yield, which can also lead to fluorescence enhancement.42,43On the other hand, uniformly dispersed AgNPs were observed in the control sample (in the presence of optimized concentrations of AChE, ATCh, AgNO3 and NaBH4) without the addition of any inhibitor (Hg2+ or Pb2+), which resulted in a decrease in emission intensity at 423 nm. This decrease may be due to the lower fluorescence quantum yield of intersystem crossing to a triplet state, which causes energy transfer to the metal, and thereby is attributed to the heavy atom effect of NPs.44,45 Stable AgNPs can induce their vibrational and rotational speed in colloidal solution and increase their Brownian motion, which increases the repulsive force between the NPs that can lead to a decrease in the external energy transfer rate.46
3.3. Fluorimetric determination of Hg2+
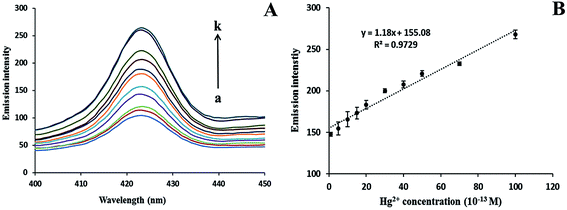
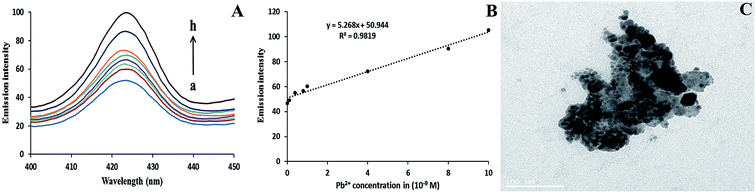
The spectral change in the fluorescence intensity of AgNPs has been observed in the reaction mixture (0.1 mM of AgNO3 and 1 mM of NaBH4), in the presence and absence of AChE, ATCh, or Hg2+ alone. In Fig. 4A (curve A), the addition of AgNO3 and NaBH4 alone could not form stable NPs and similarly, the addition of the AChE (curve B), ATCh (curve C), or Hg2+ (curve E) alone to the reaction mixture resulted in the formation of less stable NPs compared to the more stable AgNPs (curve E) formed in the presence of both AChE and ATCh. However, the addition of Hg2+ (1 × 10−9 M) to the reaction mixture in the presence of AChE and ATCh resulted in an increase in fluorescence intensity (curve F). The addition of Hg2+ to the system causes changes in the morphology of NPs, and the formed spherical NPs were observed to come closer by decreasing the enzymatic generation of thiol groups, which act as the stabilizing agent, and thereby the nano-sized particles tended to clump together, as shown in Fig. 4B.The detection of Hg2+ was carried out under optimized conditions during the formation of AgNPs in the presence of both AChE and ATCh. The emission intensity of AgNPs increases with the increase in concentration of Hg2+ (0 to 100 × 10−13 M). The increase in emission intensity is proportional to the increase in the size of AgNPs and increase in concentration of Hg2+, which inhibits the active sites of AChE. Compared to the control sample (absence of Hg2+ in the system), the addition of Hg2+ significantly increases the emission intensities of AgNPs at 423 nm, as shown in Fig. 5A. This is because the increase in the concentration of Hg2+ from 10 to 100 × 10−13 M caused an increase in the size of NP diameter by hindering the stabilizing force on the surface of AgNPs (Fig. 4B). A good linearity (R2 = 0.9729) was found between different concentrations of Hg2+ (0 to 100 × 10−13 M) and emission intensity of AgNPs at 423 nm as shown in Fig. 5B, and the limit of detection (LOD) was found to be 1.47 × 10−14 M (S/N = 3). The obtained LOD for Hg2+ in the present work is compared with the earlier reported papers, and is given in Table 1. It can be seen from Table 1 that the lowest detection limit was attained in the present study for Hg2+ detection when compared with other previous methods. The increase in fluorescence intensity for respective Hg2+ concentrations in the range (0 to 100 × 10−13 M) was examined for statistical analysis using one-way ANOVA (analysis of variance), and a p-value <0.005 was found to ensure the sensitivity of the system.
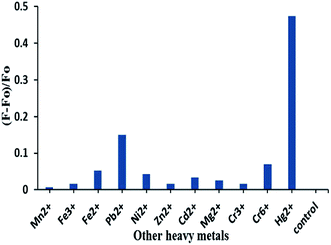
One of the principal objectives of the present study was to determine trace amounts of Hg2+ in aqueous solution with other common interfering substances. Fig. 6 shows the emission spectra of AgNPs formed during the addition of AChE and ATCh to the system in the presence of 1 × 10−9 M of different metal ions, namely, Mn2+, Fe3+, Fe2+, Pb2+, Ni2+, Zn2+, Cd2+, Mg2+, Cr3+, Cr2+, and Hg2+ (1 × 10−11 M). The emitted fluorescence intensity of AgNPs can be enhanced with other common interfering substances by resonance energy transfer, salt-induced aggregation, and charge transfer. Such phenomena may affect the specificity of this sensor for Hg2+ when compared with other metal ions. However, the presence of other metal ions in the system does not result in a significant increase in the fluorescence intensity compared to Hg2+. These results indicated that the developed sensor has a significant selectivity for Hg2+. The specificity of the current work by an enzyme-based inhibition method demonstrated greater specificity for the detection of heavy metal ions. The enzyme selectively binds with Hg2+ in comparison to other heavy metals (Hg, Pb, Fe, Ni, and Cr) as understood by comparing the degrees of aggregation of nanoparticles based on the fluorescence emission intensities. This is due to the fact that Hg2+ shows more thiophilic attraction towards the enzyme than other metal ions.14 In other words, among all the heavy metals except Hg2+, Pb2+ also showed a slight increase in fluorescence emission intensity. Based on this concept, a sensor model for the detection of Pb2+ during the synthesis of AgNPs in the presence of AChE and ATCh can be developed.
3.4. Fluorimetric determination of Pb2+
The fluorimetric detection of Pb2+ was performed in a similar manner to that of Hg2+ detection. The fluorescence increase in Pb2+ was observed in the system containing AgNO3, NaBH4, AChE, and ATCh, and the gradual increase in fluorescence intensity at 423 nm was observed in the system during the addition of different concentrations of Pb2+. Increasing concentrations of Pb2+ in the system lead to an increase in the diameter of the formed AgNPs in the presence of AChE and ATCh, and it was found that Pb2+ requires a higher duration of incubation time than Hg2+. In addition, the sensitivity of the system for Pb2+ is very low compared with the sensitivity for Hg2+. This is because the enzymatic activity of AChE in the system is extremely hindered by Hg2+ as it has a very high affinity to the thiol group (–SH) compared to Pb2+. The reaction mixtures with each concentration of Pb2+ (0.1 to 10 × 10−9 M) were incubated for 20 min at 37 ± 2 °C. Afterward, the emission intensity of each sample was recorded, as shown in Fig. 7A. A linear calibration curve (R2 = 0.9819) was observed when a graph was plotted of the emission intensity of AgNPs at 423 nm and different concentrations of Pb2+ in the range from 0.1 to 10 × 10−9 M, as shown in Fig. 7B. The limit of detection (LOD) for Pb2+ was calculated to be 0.324 × 10−10 M with a signal-to-noise ratio of 3. The addition of Pb2+ (1 × 10−9 M) to the system can also trigger the formation of nano-sized aggregates of AgNPs (Fig. 7C).3.5. Detection of Hg2+ and Pb2+ separately in real samples
The practical application of the developed sensor was estimated by determining the concentrations of Hg2+ and Pb2+ in tap and lake water samples, respectively. The detection of Hg2+ in both tap and lake water was performed by analyzing the Hg2+ content with the proposed method after spiking three different concentrations, 5, 10, and 60 × 10−13 M, of Hg2+. The increase in emission intensity at 423 nm has confirmed the presence of Hg2+ in tap and lake water (Table 3). Similarly, various concentrations of Pb2+ (0.5, 1, and 5 × 10−9 M) spiked in real samples were examined, and the increase in emission intensity at 423 nm confirmed the presence of Pb2+ in tap and lake water (Table 4). The spiked and measured values were found to be satisfactory for both Hg2+ and Pb2+. The recovery and relative standard deviation (RSD) values for both spiked Hg2+ and Pb2+ in tap water and lake water are given in Tables 3 and 4, respectively. The RSD and recovery values suggested that the present method can be used for the detection of Hg2+ or Pb2+ alone in real samples.| Sample | Added (10−13 M) | Found (10−13 M) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 5 | 4.91 | 98.20 | 0.18 |
| 10 | 9.95 | 99.50 | 0.64 | |
| 60 | 58.91 | 98.18 | 1.57 | |
| Lake water | 5 | 4.79 | 95.80 | 0.57 |
| 10 | 9.75 | 97.50 | 0.42 | |
| 60 | 55.51 | 92.52 | 2.89 |
| Sample | Added (10−9 M) | Found (10−9 M) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 0.5 | 0.52 | 104 | 0.66 |
| 1 | 1 | 100 | 0.63 | |
| 5 | 4.86 | 97.20 | 0.46 | |
| Lake water | 0.5 | 0.51 | 102 | 0.65 |
| 1 | 1.01 | 101 | 0.38 | |
| 5 | 4.56 | 91.20 | 0.47 |
3.6. Concurrent detection of Hg2+ and Pb2+
In the above method, the fluorescence detection of both Hg2+ and Pb2+ can be carried out during the synthesis of AgNPs with AChE and ATCh. The addition of Hg2+ or Pb2+ alone to the thiol-stabilized AgNPs results in an increase in the fluorescence intensity due to increase in the size of NPs during synthesis, and this will be proportional to the inhibition of AChE. This method can lead to the significant development of a sensor for the quantitative analysis of Hg2+ and Pb2+ in aqueous samples.The concurrent detection of Hg2+ was performed by keeping a fixed concentration of Pb2+ (2 × 10−9 M) throughout the experiment. The change in the fluorescence emission intensity was observed upon addition of different concentrations of Hg2+ (0 to 100 × 10−13 M) in the reaction mixtures, containing Pb2+, AgNO3, NaBH4, AChE, and ATCh. Fig. 8A reveals that the presence of Pb2+ along with various concentrations of Hg2+ can also enhance the fluorescence emission intensity at 423 nm. This could happen either because Hg2+ ions have greater tendency to adsorb on the surface of the thiol-stabilized AgNPs14 or it could be due to the higher efficiency of Hg2+ to inhibit the active site of AChE compared to Pb2+ metal ions. This process could induce the aggregation of AgNPs by increasing the electrostatic interaction due to the formation of the Hg–SR bond and by decreasing the repulsive force between thiol-stabilized AgNPs. Therefore, there is an increase in the external energy transfer rate as the concentration of Hg2+ increases, which leads to a gradual increase in the fluorescence emission intensity of AgNPs. Thus, a good linear calibration curve (R2 = 0.9585) was obtained when the emission intensity at 423 nm was plotted against different concentrations of Hg2+ with a fixed concentration of Pb2+ as shown in Fig. 8B.
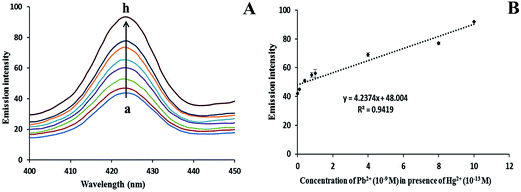
Similarly, the concurrent detection of Pb2+ was measured by keeping a fixed concentration of Hg2+ (1 × 10−13 M) throughout the experiment. Fig. 9A shows an increase in the fluorescence emission intensity at 423 nm in the presence of Hg2+ (fixed) with different concentrations of Pb2+ (0 to 10 × 10−9 M) in the system. Here, the probability of AChE inhibition by Hg2+ is much less when a lower concentration of Hg2+ is used when compared with Pb2+ concentration in the system. It was observed that the fluorescence of AgNPs was enhanced due to an increase in the size of AgNPs in the presence of both Pb2+ and Hg2+ also. The fluorescence intensity at 423 nm was found to increase with increasing concentrations of Pb2+ while keeping a fixed concentration of Hg2+ (Fig. 9A). A linear calibration curve (R2 = 0.9419) was plotted for fluorescence emission intensity of AgNPs versus different concentrations of Pb2+ with a fixed concentration of Hg2+, as shown in Fig. 9B.
4. Conclusion
The present work demonstrated that the enzymatic reaction-based stabilization of silver nanoparticles can serve as a fluorimetric probe, which allows the sensitive and selective detection of Hg2+ and Pb2+ in aqueous solutions as well as in real samples. Using fluorescence spectroscopy, the developed method can determine the inhibition in the enzymatic reaction in the presence of Hg2+ or Pb2+, which can lead to an increase in the emission intensity of silver nanoparticles at 423 nm. The determination of Hg2+ and Pb2+ was carried out based on the emission intensity of AgNPs with an inexpensive and simple assay.This method permits very high sensitivity compared with the other reported methods and can measure low concentrations of Hg2+ and Pb2+ with detection limits of 1.47 × 10−14 M and 0.324 × 10−10 M, respectively. The use of a biological recognition molecule (enzyme) enables enhanced specificity towards the interaction with the heavy metals. The developed method is simple and requires less time for the detection of Hg2+ and Pb2+. The nanoparticles can be synthesized directly without the requirement of any external stabilizing agent in the presence of an enzyme-based reaction. Further, this concept can also be used to perform the concurrent detection of Hg2+ in the presence of Pb2+ or vice versa. However, the method has its limitations also. The enzyme is expensive and it requires specific storage condition for better enzyme activity. Additionally, the potential practical applications of the concurrent detection method would require a more detailed study into overcoming the possible effects of co-existing Hg2+ and Pb2+ in the real samples, on the enzymatic interactions during the synthesis of AgNPs. The proposed sensing strategy could also be used for the detection of other inhibitor heavy metals using the enzymatic-based formation of silver nanoparticles.
Acknowledgements
The authors would like to acknowledge funding from the Defence Research & Development Organization (ERIP/ER/1103964/M/01/1485), Ministry of Defence, Government of India. We also acknowledge the VIT University for providing TEM facility.References
- F. Kandeler, C. Kampichler and O. Horak, Biol. Fertil. Soils, 1996, 23, 299–306 CrossRef.
- C. Su, L. Jiang and W. Zhang, Environmental Skeptics and Critics, 2014, 3, 24–38 Search PubMed.
- M. A. Barakat, Arabian J. Chem., 2011, 4, 361–377 CrossRef CAS.
- F. Arduini, F. Ricci, I. Bourais, A. Amine, D. Moscone and G. Palleschi, Anal. Lett., 2005, 38, 1703–1719 CrossRef CAS.
- T. W. Clarkson, L. Magos and G. J. Myers, N. Engl. J. Med., 2003, 349, 1731–1737 CrossRef CAS PubMed.
- J. Wang, B. Deng, H. Chen, X. Wang and J. Zheng, Environ. Sci. Technol., 2009, 43, 5223–5228 CrossRef CAS PubMed.
- B. V. Tangahu, S. R. Sheikh Abdullah, H. Basri, M. Idris, N. Anuar and M. Mukhlisin, Int. J. Chem. Eng., 2011, 2011, 1–31 CrossRef.
- S. M. Maliyekkal, K. P. Lisha and T. Pradeep, J. Hazard. Mater., 2010, 181, 986–995 CrossRef CAS PubMed.
- R. Singh, N. Gautam, A. Mishra and R. Gupta, Indian J. Pharmacol., 2011, 43, 246–253 CrossRef CAS PubMed.
- M. Ghaedi, M. Reza Fathi, A. Shokrollahi and F. Shajarat, Anal. Lett., 2006, 39, 1171–1185 CrossRef CAS.
- L. Rahman, W. Corns, D. Bryce and P. Stockwell, Talanta, 2000, 52, 833–843 CrossRef CAS PubMed.
- C. Huang and B. Hu, Spectrochim. Acta, Part B, 2008, 63, 437–444 CrossRef.
- W. Yantasee, Y. Lin, T. S. Zemanian and G. E. Fryxell, Analyst, 2003, 128, 467–472 RSC.
- Y. Guo, Z. Wang, W. Qu, H. Shao and X. Jiang, Biosens. Bioelectron., 2011, 26, 4064–4069 CrossRef CAS PubMed.
- A. Hamza, A. Bashammakh, A. Al-Sibaai, H. Al-Saidi and M. El-Shahawi, J. Hazard. Mater., 2010, 178, 287–292 CrossRef CAS PubMed.
- A. Fan, Y. Ling, C. Lau and J. Lu, Talanta, 2010, 82, 687–692 CrossRef CAS PubMed.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- C.-C. Huang and H.-T. Chang, Anal. Chem., 2006, 78, 8332–8338 CrossRef CAS PubMed.
- H. Lu, Y. Tang, W. Xu, D. Zhang, S. Wang and D. Zhu, Macromol. Rapid Commun., 2008, 29, 1467–1471 CrossRef CAS.
- S.-J. Liu, H.-G. Nie, J.-H. Jiang, G.-L. Shen and R.-Q. Yu, Anal. Chem., 2009, 81, 5724–5730 CrossRef CAS PubMed.
- I.-B. Kim and U. H. Bunz, J. Am. Chem. Soc., 2006, 128, 2818–2819 CrossRef CAS PubMed.
- X. Liu, Y. Tang, L. Wang, J. Zhang, S. Song, C. Fan and S. Wang, Adv. Mater., 2007, 19, 1471–1474 CrossRef CAS.
- Y. Xiao, A. A. Rowe and K. W. Plaxco, J. Am. Chem. Soc., 2007, 129, 262–263 CrossRef CAS PubMed.
- J. Liu and Y. Lu, Angew. Chem., 2007, 119, 7731–7734 CrossRef.
- C. C. Huang, Z. Yang, K. H. Lee and H. T. Chang, Angew. Chem., 2007, 119, 6948–6952 CrossRef.
- A. Zheng, J. Chen, G. Wu, H. Wei, C. He, X. Kai, G. Wu and Y. Chen, Microchim. Acta, 2009, 164, 17–27 CrossRef CAS.
- C. J. Murphy, A. M. Gole, S. E. Hunyadi, J. W. Stone, P. N. Sisco, A. Alkilany, B. E. Kinard and P. Hankins, Chem. Commun., 2008, 544–557 RSC.
- M. Rex, F. E. Hernandez and A. D. Campiglia, Anal. Chem., 2006, 78, 445–451 CrossRef CAS PubMed.
- Y.-F. Lee, F.-H. Nan, M.-J. Chen, H.-Y. Wu, C.-W. Ho, Y.-Y. Chen and C.-C. Huang, Anal. Methods, 2012, 4, 1709–1717 RSC.
- N. Ratnarathorn, O. Chailapakul and W. Dungchai, Talanta, 2015, 132, 613–618 CrossRef CAS PubMed.
- J. Liu and Y. Lu, J. Am. Chem. Soc., 2003, 125, 6642–6643 CrossRef CAS PubMed.
- Z. Wang, J. H. Lee and Y. Lu, Adv. Mater., 2008, 20, 3263–3267 CrossRef CAS.
- K.-W. Huang, C.-J. Yu and W.-L. Tseng, Biosens. Bioelectron., 2010, 25, 984–989 CrossRef CAS PubMed.
- S. Deo and H. A. Godwin, J. Am. Chem. Soc., 2000, 122, 174–175 CrossRef CAS.
- E. Mohamed Ali, Y. Zheng, H.-h. Yu and J. Y. Ying, Anal. Chem., 2007, 79, 9452–9458 CrossRef PubMed.
- Z. Li, Y. Wang, Y. Ni and S. Kokot, Sens. Actuators, B, 2015, 207, 490–497 CrossRef CAS.
- D. N. Kumar, A. Rajeshwari, S. Alex, M. Sahu, A. Raichur, N. Chandrasekaran and A. Mukherjee, RSC Adv., 2015, 5, 61998–62006 RSC.
- D. N. Kumar, A. Rajeshwari, S. A. Alex, N. Chandrasekaran and A. Mukherjee, New J. Chem., 2015, 39, 1172–1178 RSC.
- O. C. Compton and F. E. Osterloh, J. Am. Chem. Soc., 2007, 129, 7793–7798 CrossRef CAS PubMed.
- H. Jans, X. Liu, L. Austin, G. Maes and Q. Huo, Anal. Chem., 2009, 81, 9425–9432 CrossRef CAS PubMed.
- S. A. McFarland and N. S. Finney, J. Am. Chem. Soc., 2002, 124, 1178–1179 CrossRef CAS PubMed.
- D. Xiang, G. Zeng, K. Zhai, L. Li and Z. He, Analyst, 2011, 136, 2837–2844 RSC.
- S. Shankar and S. A. John, Sens. Actuators, B, 2015, 221, 1202–1208 CrossRef CAS.
- P. Khoza and T. Nyokong, J. Mol. Catal. A: Chem., 2014, 395, 34–41 CrossRef CAS.
- L. Balan, R. Schneider, C. Turck, D. Lougnot and F. Morlet-Savary, J. Nanomater., 2012, 2012, 1 CrossRef.
- A. Menbari, A. A. Alemrajabi and Y. Ghayeb, Energy Convers. Manage., 2016, 108, 501–510 CrossRef CAS.
- J. Xie, Y. Zheng and J. Y. Ying, Chem. Commun., 2010, 46, 961–963 RSC.
- Z. Wang, J. H. Lee and Y. Lu, Chem. Commun., 2008, 6005–6007 RSC.
- C. Guo and J. Irudayaraj, Anal. Chem., 2011, 83, 2883–2889 CrossRef CAS PubMed.
- W. Lu, X. Qin, S. Liu, G. Chang, Y. Zhang, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Anal. Chem., 2012, 84, 5351–5357 CrossRef CAS PubMed.
- L. Zhou, Y. Lin, Z. Huang, J. Ren and X. Qu, Chem. Commun., 2012, 48, 1147–1149 RSC.
- C.-W. Liu, C.-C. Huang and H.-T. Chang, Anal. Chem., 2009, 81, 2383–2387 CrossRef CAS PubMed.
- H. Wu, J. Liang and H. Han, Microchim. Acta, 2008, 161, 81–86 CrossRef CAS.
- Y.-Y. Chen, H.-T. Chang, Y.-C. Shiang, Y.-L. Hung, C.-K. Chiang and C.-C. Huang, Anal. Chem., 2009, 81, 9433–9439 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00193a |
| This journal is © The Royal Society of Chemistry 2016 |